General Information
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram-, Anticancer
-
Target Organism
-
- Gram-negative bacterium: Escherichia coli K12 (MIC=80 µM).
- Drug-resistant KBv cancer cells (LC50=39 µM), Drug-sensitive KB cancer cells (LC50=40 µM).
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Cyclic
-
N-terminal Modification
- Free
-
C-terminal Modification
- Amidation
-
Nonterminal Modifications and Unusual Amino Acids
- Disulfide bonds
-
Stereochemistry
- L
-
Structure
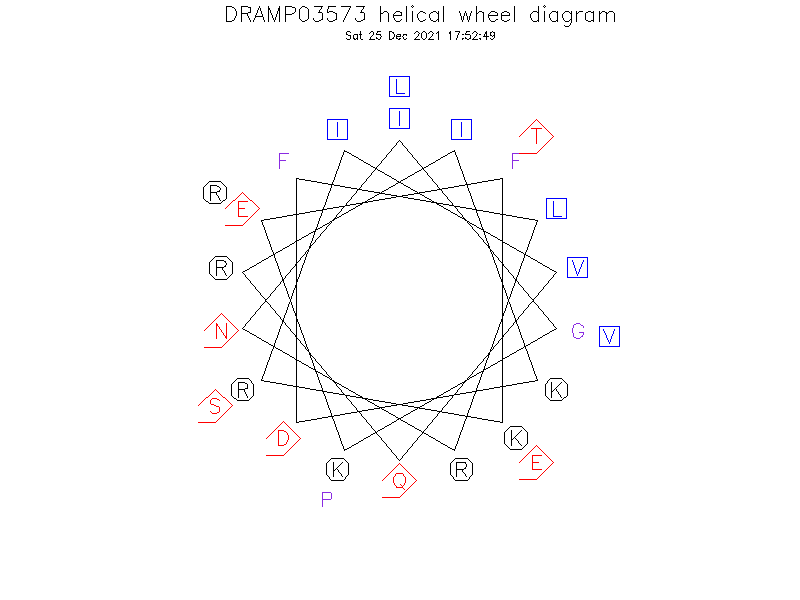
- Alpha helix (1 helices; 14 residues)
-
Structure Description
- Residues 17-29 of LL-37(13-37) are helical.
-
Helical Wheel Diagram
-
PDB ID
- 2FCG resolved by NMR.
-
Predicted Structure
- There is no predicted structure for DRAMP03573.
Physicochemical Information
-
Formula
- C137H232N42O36
Absent Amino Acids
- ACHMWY
Common Amino Acids
- R
Mass
- 3043.61
PI
- 10.93
Basic Residues
- 7
Acidic Residues
- 3
Hydrophobic Residues
- 9
Net Charge
- +4
-
Boman Index
- -77.24
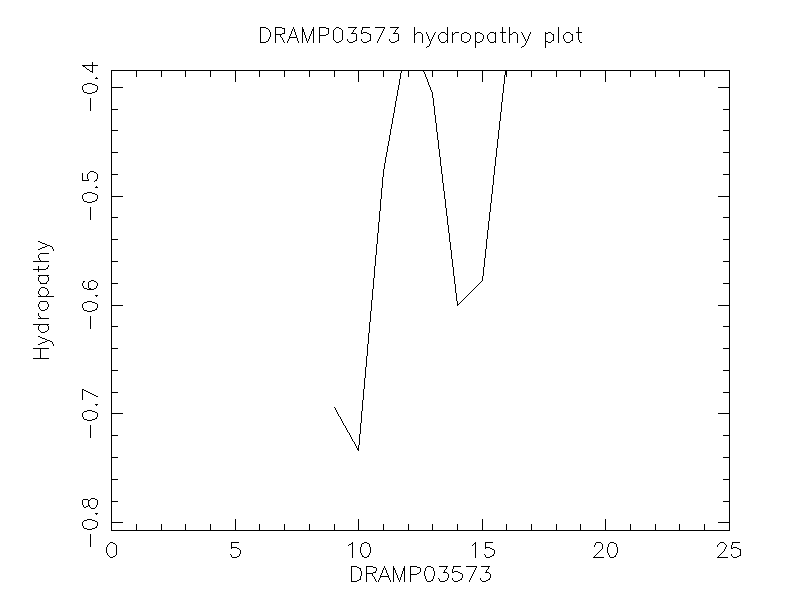
Hydrophobicity
- -0.624
Aliphatic Index
- 101.2
Half Life
-
- Mammalian:20 hour
- Yeast:30 min
- E.coli:>10 hour
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 4
DRAMP03573
Comments Information
The slightly lower activity of LL-37(13-37) may result from the interference of the disordered regions with membrane binding of the peptide.
Literature Information
- ·Literature 1
-
Title
- Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region.
-
Pubmed ID
- 16637646
-
Reference
- J Am Chem Soc. 2006 May 3;128(17):5776-5785.
-
Author
- Li X, Li Y, Han H, Miller DW, Wang G.