General Information
-
DRAMP ID
- DRAMP29091
-
Peptide Name
- Skh-AMP1
-
Source
- Satureja khuzistanica
-
Family
- Belongs to the Lamiaceae family, subfamily Nepetoideae.
-
Gene
- N/A
-
Sequence
- GRTSKQELCTWERGSVRQADKTIAG
-
Sequence Length
- 25
-
UniProt Entry
- No entry found
-
Protein Existence
- Natural peptide
Activity Information
-
Biological Activity
- Antimicrobial, Antifungal
-
Target Organism
-
- [Ref.31128493] Fungi: Candida albicans ATCC 10231 (MIC = 21.6 μM, MFC = 43.2 μM), Candida krusei DSM 70079 (MIC = 20.7 μM, MFC = 41.4 μM), Candida glabrata ATCC 90030 (MIC = 19.8 μM, MFC = 39.6 μM), Aspergillus niger ATCC 9029 (MIC = 22.5 μM, MFC = 45 μM), Aspergillus flavus PFCC 100 (MIC = 23.4 μM, MFC = 58.5 μM), Aspergillus fumigatus Af 293 (MIC = 20.7 μM, MFC = 41.4 μM)
-
Hemolytic Activity
-
- [Ref.31128493] Using human erythrocytes, the hemolytic activity of Skh-AMP1 at concentrations from 3.6 μM to 72 μM was reported in the range of 0.19%–2.1%. At the MIC concentration of 25.2 μM, a very low hemolytic activity of 0.67% (SD = 0.02) was observed. Also, this peptide at MFC concentrations (36, 54 and 72 μM) showed 0.98%, 1.45% and 2.1% hemolytic activity for human erythrocytes.
-
Cytotoxicity
-
- [Ref.31128493] Skh-AMP1 has about 3.6% cytotoxicity at the concentration of 25.2 μM in 48 h. Therefore, this peptide had no significant cytotoxic effect at the concentrations of 12.6 and 25.2 μM at 6, 24, and 48 h. However, Skh-AMP1 has about 12% and 27% cytotoxicity at the concentration of 50.4 μM and 63 μM, respectively
-
Binding Target
- Fungal membrane
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Free
-
Nonterminal Modifications and Unusual Amino Acids
- None
-
Stereochemistry
- L
-
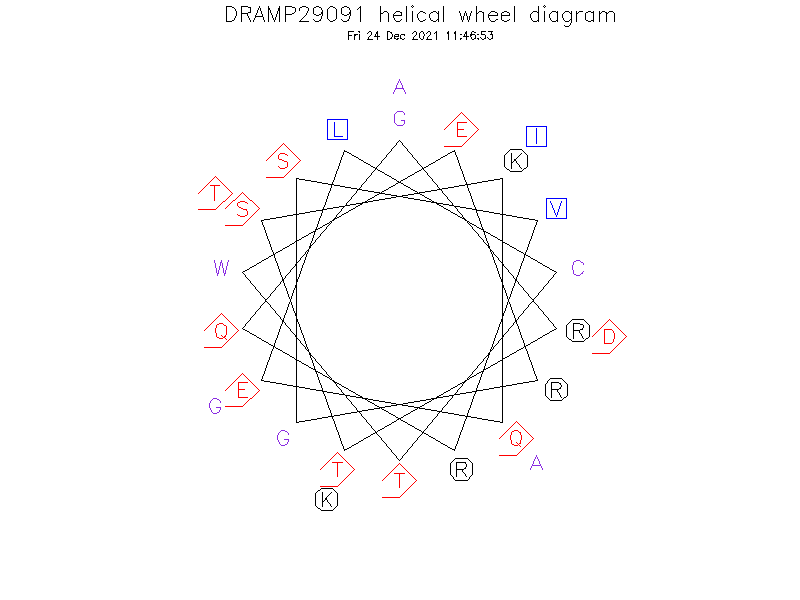
Structure
- α-helical
-
Structure Description
- Evaluation of the peptide three-dimensional structure showed that, despite the unusual distribution of amino acids in the Wheel projection, Skh-AMP1 maybe forms a α-helix structure with more hydrophobic C-terminal than that of the N-terminal.
-
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP29091.
Physicochemical Information
-
Formula
- C115H193N39O39S
Absent Amino Acids
- FHMNPY
Common Amino Acids
- GRT
Mass
- 2778.1
PI
- 9.31
Basic Residues
- 5
Acidic Residues
- 3
Hydrophobic Residues
- 6
Net Charge
- +2
-
Boman Index
- -7986
Hydrophobicity
- -1.04
Aliphatic Index
- 50.8
Half Life
-
- Mammalian:30 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 5500
Absorbance 280nm
- 229.17
Polar Residues
- 9
DRAMP29091
Comments Information
Comment
- The antifungal activity of Skh-AMP1 may be related to its ability to disrupt fungal cell membrane permeabilization and induce enhanced ROS production. Therefore, Skh-AMP1 can be introduced as a novel antifungal candidate for developing new therapeutic agents.
Literature Information
- ·Literature 1
-
Title
- Effects of the antifungal peptide Skh-AMP1 derived from Satureja khuzistanica on cell membrane permeability, ROS production, and cell morphology of conidia and hyphae of Aspergillus fumigatus.
-
Pubmed ID
- 31704210
-
Reference
- Peptides. 2020 Jan;123:170195. doi: 10.1016/j.peptides.2019.170195. Epub 2019 Nov 6.
-
Author
- Khani S, Seyedjavadi SS, Hosseini HM, Goudarzi M, Valadbeigi S, Khatami S, Ajdary S, Eslamifar A, Amani J, Imani Fooladi AA, Razzaghi-Abyaneh M
- ·Literature 2
-
Title
- Isolation and functional characterization of an antifungal hydrophilic peptide, Skh-AMP1, derived from Satureja khuzistanica leaves
-
Pubmed ID
- 31128493
-
Reference
- Phytochemistry. 2019 Aug;164:136-143. doi: 10.1016/j.phytochem.2019.05.011. Epub 2019 May 23.
-
Author
- Soghra Khani, Sima Sadat Seyedjavadi, Hadi Zare-Zardini, Hamideh Mahmoodzadeh Hosseini, Mehdi Goudarzi, Shohreh Khatami, Jafar Amani, Abbas Ali Imani Fooladi, Mehdi Razzaghi-Abyaneh