General Information
-
DRAMP ID
- DRAMP00132
-
Peptide Name
- Lactococcin G subunit alpha (Galpha; Bacteriocin)
-
Source
- Lactococcus lactis subsp. lactis (Streptococcus lactis) (Gram-positive bacteria)
-
Family
- Belongs to the class IIb bacteriocin
-
Gene
- Not found
-
Sequence
- GTWDDIGQGIGRVAYWVGKALGNLSDVNQASRINRKKKH
-
Sequence Length
- 39
-
UniProt Entry
- P36961
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial
-
Target Organism
- Lactococcus.
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
Structure
- Alpha helix
-
Structure Description
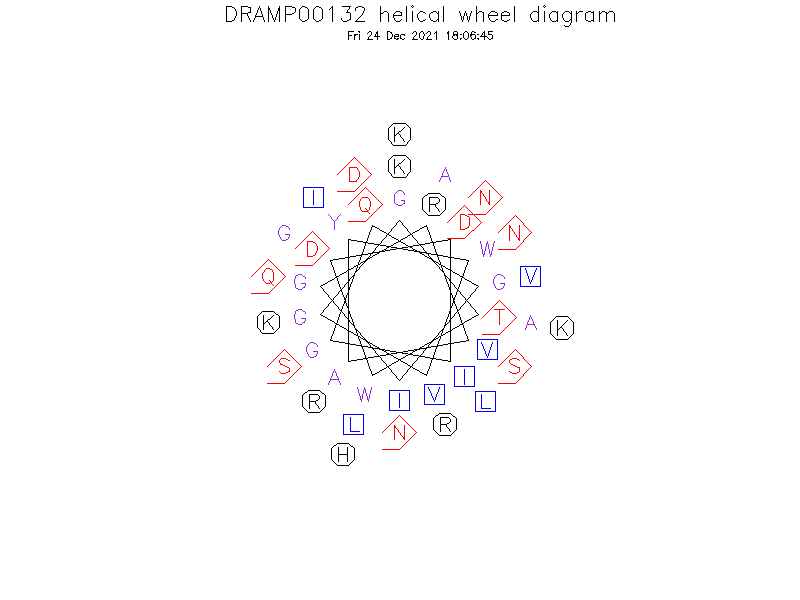
- In DPC, LcnG-alpha has an N-terminal alpha-helix (residues 3-21) that contains a GxxxG helix-helix interaction motif (residues 7-11) and a less well defined C-terminal alpha-helix (residues 24-34), and in between (residues 18-22) there is a second somewhat flexible GxxxG-motif. Its structure in TFE was similar.
-
Helical Wheel Diagram
- 2JPJ->
-
Predicted Structure
- There is no predicted structure for DRAMP00132.
Physicochemical Information
-
Formula
- C189H303N61O55
Absent Amino Acids
- CEFMP
Common Amino Acids
- G
Mass
- 4309.86
PI
- 10.16
Basic Residues
- 8
Acidic Residues
- 3
Hydrophobic Residues
- 13
Net Charge
- +5
-
Boman Index
- -85.84
Hydrophobicity
- -0.744
Aliphatic Index
- 80
Half Life
-
- Mammalian:30 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 12490
Absorbance 280nm
- 328.68
Polar Residues
- 13
DRAMP00132
Comments Information
MOA
- The N-terminal halves of both the alpha and beta peptides may form amphiphilic alpha-helices, suggesting that the peptides are pore-forming toxins that create cell membrane channels through a "barrel-stave" mechanism. Bacteriocin activity requires interaction of alpha and beta peptides in a molar ratio of 7
Literature Information
- ·Literature 1
-
Title
- A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides.
-
Pubmed ID
- 1512201
-
Reference
- J Bacteriol. 1992 Sep;174(17):5686-5692.
-
Author
- Nissen-Meyer J, Holo H, H¥varstein LS, Sletten K, Nes IF.
- ·Literature 2
-
Title
- Three-dimensional structure of the two peptides that constitute the two-peptide bacteriocin lactococcin G.
-
Pubmed ID
- 18187052
-
Reference
- Biochim Biophys Acta. 2008 Mar;1784(3):543-554.
-
Author
- Rogne P, Fimland G, Nissen-Meyer J, Kristiansen PE.