General Information
-
DRAMP ID
- DRAMP01303
-
Peptide Name
- Phylloseptin-3 (PS-3; Frogs, amphibians, animals)
-
Source
- Phyllomedusa hypochondrialis (Orange-legged leaf frog)
-
Family
- Not found
-
Gene
- psn3
-
Sequence
- FLSLIPHAINAVSALANHG
-
Sequence Length
- 19
-
UniProt Entry
- P84568
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-, Antifungal
-
Target Organism
-
- [ATCC Strains]: Gram-positive bacteria: S. aureus (MIC=32.9 µM);
- Gram-negative bacteria: Escherichia coli (MIC=65.6 µM), Klebsiella pneumoniae (MIC=65.6 µM), Pseudomonas aeruginosa (MIC=65.6 µM). NOTE: Initial inoculum of 108 cells/Ml.
- [Wild Type]: Gram-positive bacteria: S. aureus (MIC=8.2 µM), S. agalactiae (MIC=4.1 µM);
- Gram-negative bacteria: Pseudomonas aeruginosa (MIC=32.8 µM), A. calcoaceticus (MIC=4.1 µM).
- Fungi: Candida albicans (MIC=8.2 µM).
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
- No cytotoxicity information found in the reference(s) presented
-
Binding Target
- Cell membrane
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Amidation
-
Nonterminal Modifications and Unusual Amino Acids
- Free
-
Stereochemistry
- L
-
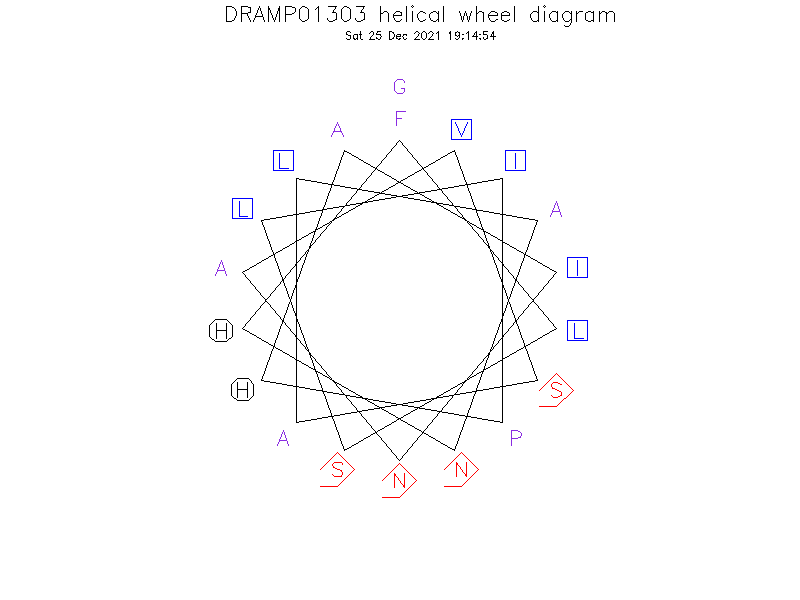
Structure
- Alpha helix (1 helices; 11 residues)
-
Structure Description
- The antimicrobial phylloseptin peptides adopt alpha-helical conformations in membrane environments which are stabilized by electrostatic interactions of the helix dipole as well as other contributions such hydrophobic and capping interactions.(Ref.2)
-
Helical Wheel Diagram
-
PDB ID
- 2JQ1 resolved by NMR.
-
Predicted Structure
- There is no predicted structure for DRAMP01303.
Physicochemical Information
-
Formula
- C89H141N25O24
Absent Amino Acids
- CDEKMQRTWY
Common Amino Acids
- A
Mass
- 1945.25
PI
- 6.92
Basic Residues
- 2
Acidic Residues
- 0
Hydrophobic Residues
- 11
Net Charge
- +2
-
Boman Index
- 10.4
Hydrophobicity
- 0.926
Aliphatic Index
- 138.95
Half Life
-
- Mammalian:1.1 hour
- Yeast:3 min
- E.coli:2 min
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 5
DRAMP01303
Comments Information
Function
- Has antimicrobial activity (By similarity).
Tissue specificity
- Expressed by the skin glands.
PTM
- C-terminal amidation.
Literature Information
- ·Literature 1
-
Title
- Phylloseptins: a novel class of anti-bacterial and anti-protozoan peptides from the Phyllomedusa genus.
-
Pubmed ID
- 15752569
-
Reference
- Peptides. 2005 Apr;26(4):565-573.
-
Author
- Leite JR, Silva LP, Rodrigues MI, Prates MV, Brand GD, Lacava BM, Azevedo RB, Bocca AL, Albuquerque S, Bloch C Jr.
- ·Literature 2
-
Title
- Solution NMR structures of the antimicrobial peptides phylloseptin-1, -2, and -3 and biological activity: the role of charges and hydrogen bonding interactions in stabilizing helix conformations.
-
Pubmed ID
- 18656510
-
Reference
- Peptides. 2008 Oct;29(10):1633-1644.
-
Author
- Resende JM, Moraes CM, Prates MV, Cesar A, Almeida FC, Mundim NC, Valente AP, Bemquerer MP, Piló-Veloso D, Bechinger B.