General Information
-
DRAMP ID
- DRAMP03222
-
Peptide Name
- M-ctenitoxin-Cs1a (M-CNTX-Cs1a; Cupiennin-1a; spiders, Arthropods, animals)
-
Source
- Cupiennius salei (Wandering spider)
-
Family
- Belongs to the cupiennin family
-
Gene
- Not found
-
Sequence
- GFGALFKFLAKKVAKTVAKQAAKQGAKYVVNKQME
-
Sequence Length
- 35
-
UniProt Entry
- P83619
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-, Insecticidal
-
Target Organism
-
- [Ref.11792701]Gram-negative bacteria: Escherichia coli ATCC 25922 (MIC=0.31-0.63 µM), Pseudomonas aeruginosa ATCC 27853 (MIC=0.31-0.63 µM);
- Gram-positive bacteria: Staphylococcus aureus ATCC 29213 (MIC=0.31-0.63 µM), Enterococcus faecalis ATCC 29212 (MIC=2.50-5.00 µM).
-
Hemolytic Activity
-
- [Ref.11792701]EC50=24.4 μM against human red blood cells.
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- calcium calmodulin
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Amidation
-
Nonterminal Modifications and Unusual Amino Acids
- Free
-
Stereochemistry
- L
-
Structure
- Alpha helix (3 helices; 11 residues)
-
Structure Description
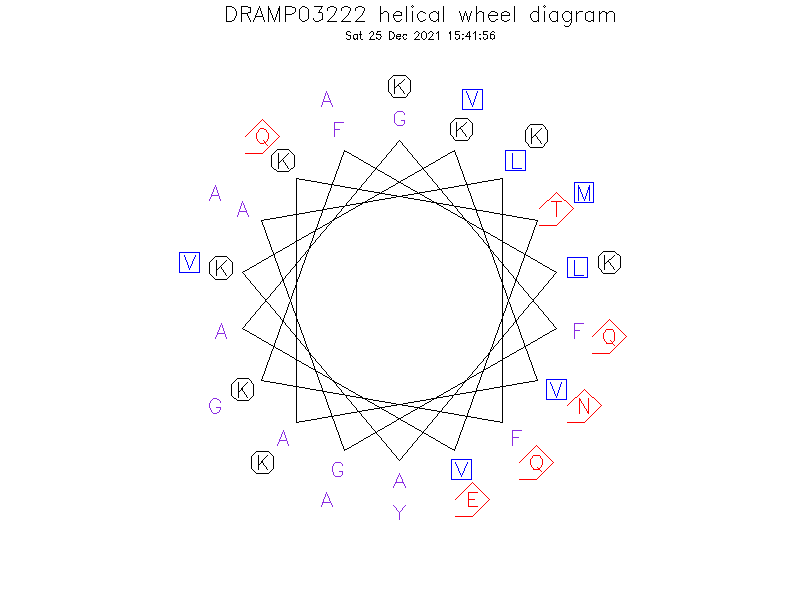
- The peptide adopts a helix-hinge-helix structure in a membrane mimicking solvent. The hinge may play a role in allowing the amphipathic N-terminal helix and polar C-terminal helix to orient independently upon membrane binding, in order to achieve maximal antibacterial efficacy.(Ref.2)
-
Helical Wheel Diagram
-
PDB ID
- 2K38 resolved by NMR.
-
Predicted Structure
- There is no predicted structure for DRAMP03222.
Physicochemical Information
-
Formula
- C176H289N47O44S
Absent Amino Acids
- CDHIPRSW
Common Amino Acids
- K
Mass
- 3799.58
PI
- 10.3
Basic Residues
- 8
Acidic Residues
- 1
Hydrophobic Residues
- 16
Net Charge
- +7
-
Boman Index
- -24.4
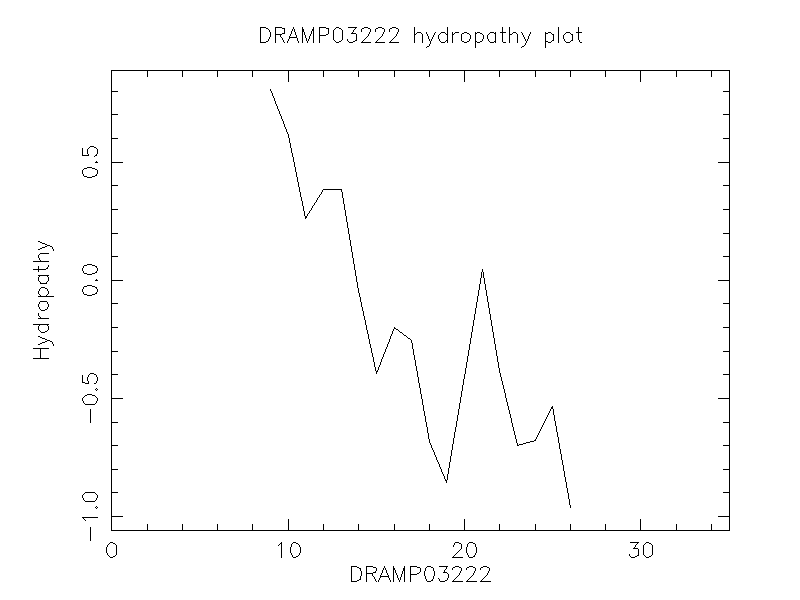
Hydrophobicity
- -0.131
Aliphatic Index
- 75.43
Half Life
-
- Mammalian:30 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 1490
Absorbance 280nm
- 43.82
Polar Residues
- 6
DRAMP03222
Comments Information
Function
- Has antimicrobial activity. Has insecticidal and hemolytic activities. Probably acts by disturbing membrane Function
Literature Information
- ·Literature 1
-
Title
- Cupiennin 1, a new family of highly basic antimicrobial peptides in the venom of the spider Cupiennius salei (Ctenidae).
-
Pubmed ID
- 11792701
-
Reference
- J Biol Chem. 2002 Mar 29;277(13):11208-11216.
-
Author
- Kuhn-Nentwig L, Muller J, Schaller J, Walz A, Dathe M, Nentwig W.
- ·Literature 2
-
Title
- Solution structure and interaction of cupiennin 1a, a spider venom peptide, with phospholipid bilayers.
-
Pubmed ID
- 17319697
-
Reference
- Biochemistry. 2007 Mar 20;46(11):3576-3585.
-
Author
- Pukala TL, Boland MP, Gehman JD, Kuhn-Nentwig L, Separovic F, Bowie JH.