General Information
-
DRAMP ID
- DRAMP20771
-
Peptide Name
- Acanthaporin (parasite, amoebozoa, protozoa, protists)
-
Source
- Acanthamoeba culbertsoni
-
Family
- Not found
-
Gene
- Not found
-
Sequence
- GKCSVLKKVACAAAIAGAVAACGGIDLPCVLAALKAAEGCASCFCEDHCHGVCKDLHLC
-
Sequence Length
- 59
-
UniProt Entry
- I3NI56
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-
-
Target Organism
- No MICs found in DRAMP database
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
Structure
- Alpha helix
-
Structure Description
- Not found
-
Helical Wheel Diagram
-
Predicted Structure
- There is no predicted structure for DRAMP20771.
Physicochemical Information
-
Formula
- C244H404N70O72S10
Absent Amino Acids
- MNQRTWY
Common Amino Acids
- A
Mass
- 5790.92
PI
- 6.94
Basic Residues
- 8
Acidic Residues
- 5
Hydrophobic Residues
- 27
Net Charge
- +3
-
Boman Index
- 1620
Hydrophobicity
- 0.878
Aliphatic Index
- 99.49
Half Life
-
- Mammalian:30 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 625
Absorbance 280nm
- 10.78
Polar Residues
- 18
DRAMP20771
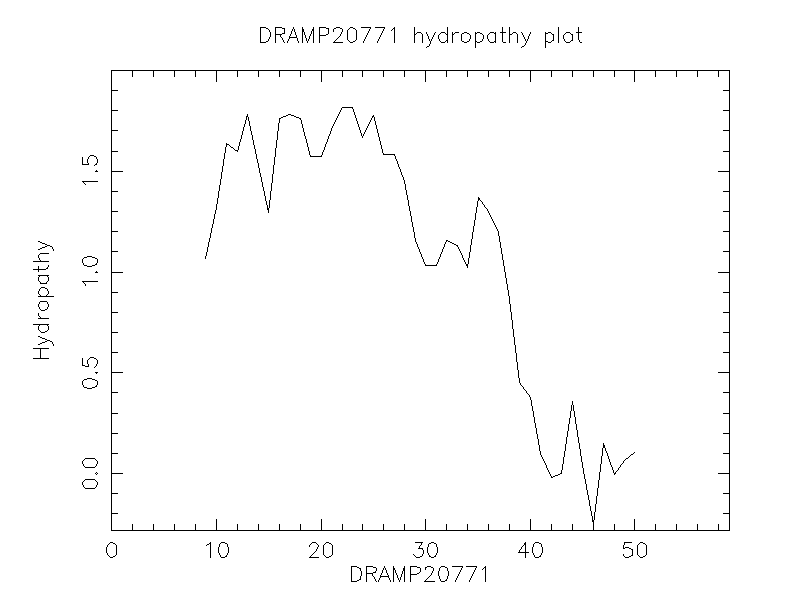
Comments Information
Function
- Acanthaporin is antimicrobially active against several Gram-positive and Gram-negative bacteria at pH 5.2 but is inactive at pH 7.4, reflecting a pH-dependent activity of the protein.
Literature Information
- ·Literature 1
-
Title
- Structure and function of a unique pore-forming protein from a pathogenic acanthamoeba.
-
Pubmed ID
- 23143413
-
Reference
- Nat Chem Biol. 2013 Jan;9(1):37-42.
-
Author
- Michalek M, Sönnichsen FD, Wechselberger R, Dingley AJ, Hung CW, Kopp A, Wienk H, Simanski M, Herbst R, Lorenzen I, Marciano-Cabral F, Gelhaus C, Gutsmann T, Tholey A, Grötzinger J, Leippe M.