General Information
-
DRAMP ID
- DRAMP29097
-
Peptide Name
- SHβAP
-
Source
- Katsuwonus pelamis
-
Family
- Not found
-
Gene
- Not found
-
Sequence
- TQQAFQKFLAAVTSALGKQYH
-
Sequence Length
- 21
-
UniProt Entry
- No entry found
-
Protein Existence
- Not found
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial,Anti-Gram+,Anti-Gram-,Antifungal
-
Target Organism
-
- [Ref.24495783]Gram-positive bacteria:
Target Organism Activity B.subtilis KCTC1021(MEC=16.0 μg/mL); B.subtilis RM125(MEC=6.5 μg/mL); S.aureus KCTC1621(MEC=57.0 μg/mL);S.iniae FP5229(MEC=23.0 μg/mL); - - Gram-negative bacteria:
Target Organism Activity E.coli D31(MEC=15.0 μg/mL); E.coli KCTC1116(MEC=2.6 μg/mL); E.coli ML35p(MEC=6.2 μg/mL); P.aeruginosa KCTC2004(MEC=19.0 μg/mL); S.enterica KCTC2514(MEC=2.0 μg/mL); S.sonnei KCTC2009(MEC=6.0 μg/mL);A.hydrophila KCTC2358(MEC>125.0 μg/mL); V.parahaemolyticus HUFP91(MEC=1.9 μg/mL); V.parahaemolyticus KCCM41664(MEC=1.5 μg/mL); - - Yeast: C.albicans KCTC7965(MEC=12.0 μg/mL).
- [Ref.24495783]Gram-positive bacteria:
-
Hemolytic Activity
-
- [Ref.24495783]not cause hemolysis against RBCs up to 100 μg/mL concentration.
-
Cytotoxicity
- No cytotoxicity information found in the reference(s) presented
-
Binding Target
- liposomes
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Free
-
Nonterminal Modifications and Unusual Amino Acids
- None
-
Stereochemistry
- L
-
Structure
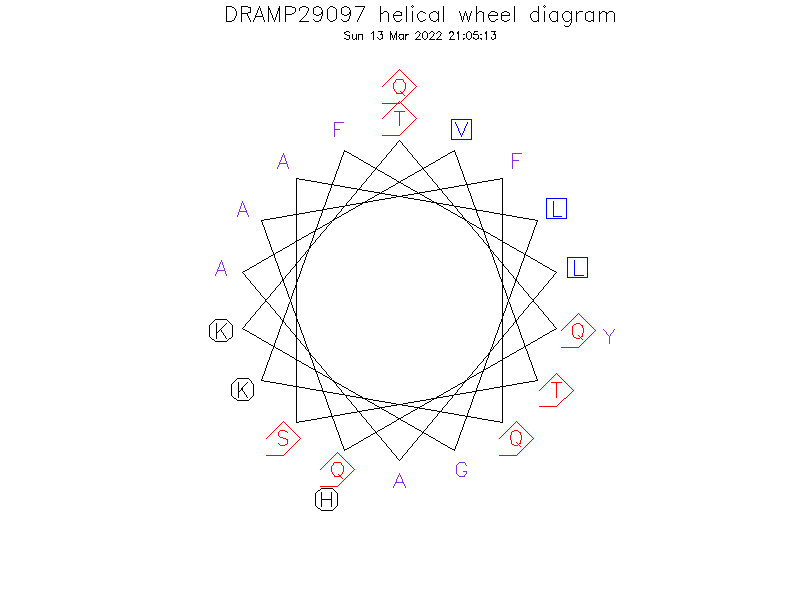
- Alpha helix
-
Structure Description
- Not found
-
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP29097.
Physicochemical Information
-
Formula
- C107H165N29O30
Absent Amino Acids
- CDEIMNPRW
Common Amino Acids
- AQ
Mass
- 2337.66
PI
- 9.7
Basic Residues
- 3
Acidic Residues
- 0
Hydrophobic Residues
- 9
Net Charge
- +3
-
Boman Index
- -1858
Hydrophobicity
- -0.205
Aliphatic Index
- 70
Half Life
-
- Mammalian:7.2 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 1490
Absorbance 280nm
- 74.5
Polar Residues
- 5
DRAMP29097
Comments Information
Function
- SHβAP showed broad spectrum antimicrobial activity without hemolytic activity.Antimicrobial activity of the peptide was heatstable and pH resistant but is sensitive to proteases and salt.The action mode of SHβAP is bacteriostatic process rather than bactericidal one.
Literature Information
- ·Literature 1
-
Title
- Antimicrobial function of SHβAP, a novel hemoglobin β chain-related antimicrobial peptide, isolated from the liver of skipjack tuna, Katsuwonus pelamis.
-
Pubmed ID
- 24495783
-
Reference
- Fish Shellfish Immunol. 2014 Mar;37(1):173-83.
-
Author
- Seo JK, Lee MJ, Jung HG, Go HJ, Kim YJ, Park NG.