General Information
-
DRAMP ID
- DRAMP00013
-
Peptide Name
- Lantibiotic lichenicidin VK21 A2 (LchA2; Lchbeta; Bacteriocin)
-
Source
- Bacillus licheniformis (strain ATCC 14580/VK21/DSM 13) (Gram-positive bacteria)
-
Family
- Belongs to the lantibiotic family (Class I bacteriocin)
-
Gene
- lchA2
-
Sequence
- TTPATTSSWTCITAGVTVSASLCPTTKCTSRC
-
Sequence Length
- 32
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial
-
Target Organism
-
- Only Lchbeta: Bacillus megaterium VKM41 (IC50=2 uM), Bacillus subtilis L1 (IC50=30 uM), Rhodococcus sp. SS2 (IC50=16.6 uM), Micrococcus luteus B1314 (IC50=2.6 uM), Staphylococcus aureus 209p (IC50=20 uM).
- Lchalpha+Lchbeta: Bacillus megaterium VKM41 (IC50=0.12 uM), Bacillus subtilis L1 (IC50=0.64 uM), Rhodococcus sp. SS2 (IC50=0.64 uM), Micrococcus luteus B1314 (IC50=0.09 uM), Staphylococcus aureus 209p (IC50=0.64 uM).
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Cell membrane
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
Structure
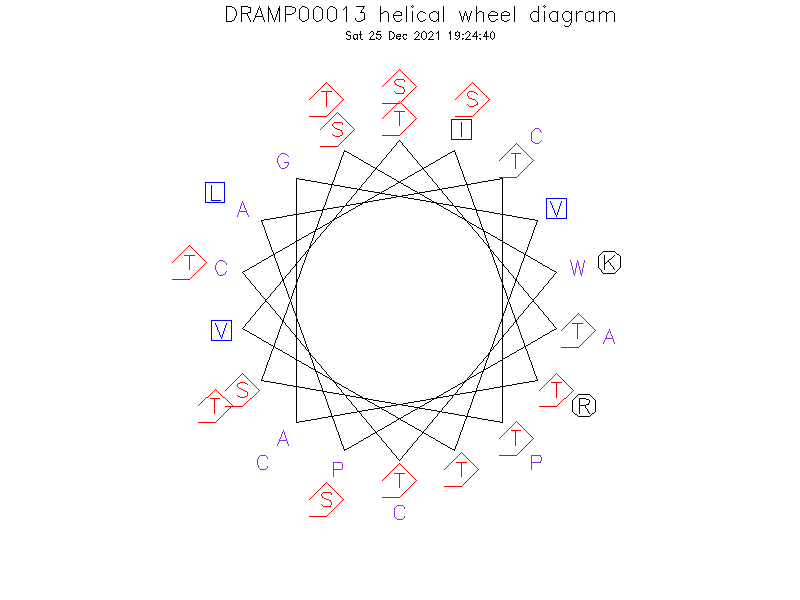
- Alpha helix (1 helices; 14 residues)
-
Structure Description
- The Lchbeta peptide represents a prolonged hydrophobic alpha-helix flanked with more flexible N- and C-terminal domains.
-
Helical Wheel Diagram
-
PDB ID
- 2KTO resolved by NMR.
-
Predicted Structure
- There is no predicted structure for DRAMP00013.
Physicochemical Information
-
Formula
- C133H223N37O48S4
Absent Amino Acids
- DEFHMNQY
Common Amino Acids
- T
Mass
- 3236.69
PI
- 8.52
Basic Residues
- 2
Acidic Residues
- 0
Hydrophobic Residues
- 8
Net Charge
- +2
-
Boman Index
- -31.43
Hydrophobicity
- 0.256
Aliphatic Index
- 51.88
Half Life
-
- Mammalian:7.2 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 5750
Absorbance 280nm
- 185.48
Polar Residues
- 20
DRAMP00013
Comments Information
Function
- The mature peptides, Lchalpha and Lchbeta, interact synergistically to possess antibiotic activity against Gram-positive bacteria within a nanomolar concentration range, though the individual peptides were shown to be active at micromolar concentrations. The bactericidal activity of lantibiotics is based on depolarization of energized bacterial cytoplasmic membranes, initiated by the formation of aqueous transmembrane pores. Combined LchA1 and LchA2 peptides also inhibit Bacillus sp. HIL-Y85/54728, L.lactis DPC3417 and B.halodurans C-125, which produce lantibiotics themselves. Inactivated by proteinase K and pronase E, but not by trypsin and chymotrypsin.
PTM
- Maturation of lantibiotics involves the enzymic conversion of Thr, and Ser into dehydrated AA and the formation of thioether bonds with cysteine. This is followed by membrane translocation and cleavage of the modified precursor.
Literature Information
- ·Literature 1
-
Title
- Identification of a novel two-peptide lantibiotic, lichenicidin, following rational genome mining for LanM proteins.
-
Pubmed ID
- 19561184
-
Reference
- Appl Environ Microbiol. 2009 Sep;75(17):5451-5460.
-
Author
- Begley M, Cotter PD, Hill C, Ross RP.
- ·Literature 2
-
Title
- Production of the novel two-peptide lantibiotic lichenicidin by Bacillus licheniformis DSM 13.
-
Pubmed ID
- 19707558
-
Reference
- PLoS One. 2009 Aug 26;4(8):e6788.
-
Author
- Dischinger J, Josten M, Szekat C, Sahl HG, Bierbaum G.
- ·Literature 3
-
Title
- Isolation, structure elucidation, and synergistic antibacterial activity of a novel two-component lantibiotic lichenicidin from Bacillus licheniformis VK21.
-
Pubmed ID
- 20578714
-
Reference
- Biochemistry. 2010 Aug 3;49(30):6462-6472.
-
Author
- Shenkarev ZO, Finkina EI, Nurmukhamedova EK, Balandin SV, Mineev KS, Nadezhdin KD, Yakimenko ZA, Tagaev AA, Temirov YV, Arseniev AS, Ovchinnikova TV.