General Information
-
DRAMP ID
- DRAMP00028
-
Peptide Name
- Lantibiotic epidermin (Bacteriocin)
-
Source
- Staphylococcus epidermidis TU 3298 / DSM 3095 (Gram-positive bacteria)
-
Family
- Belongs to the type A lantibiotic family (Class I bacteriocin)
-
Gene
- epiA
-
Sequence
- IASKFICTPGCAKTGSFNSYCC
-
Sequence Length
- 22
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial
-
Target Organism
- No MICs found in DRAMP database
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Lipid II
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
Structure
- Rich
-
Structure Description
- Not found
-
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP00028.
Physicochemical Information
-
Formula
- C99H153N25O30S4
Absent Amino Acids
- DEHLMQRVW
Common Amino Acids
- C
Mass
- 2301.69
PI
- 8.52
Basic Residues
- 2
Acidic Residues
- 0
Hydrophobic Residues
- 6
Net Charge
- +2
-
Boman Index
- -6.8
Hydrophobicity
- 0.427
Aliphatic Index
- 44.55
Half Life
-
- Mammalian:20 hour
- Yeast:30 min
- E.coli:>10 hour
Extinction Coefficient Cystines
- 1740
Absorbance 280nm
- 82.86
Polar Residues
- 13
DRAMP00028
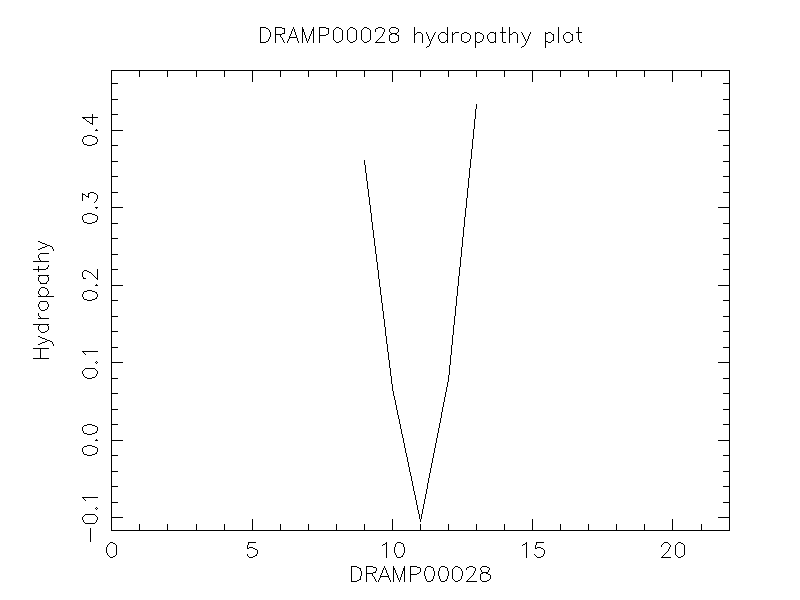
Comments Information
Function
- Lanthionine-containing peptide antibiotic (lantibiotic) active on Gram-positive bacteria. The bactericidal activity of lantibiotics is based on depolarization of energized bacterial cytoplasmic membranes, initiated by the formation of aqueous transmembrane pores.
PTM
- There are one didehydrobutyrine (T14), two lanthionines (S3-C7; S16-C21) and one Beta-methyllanthionine (T8-C11) and one S-(2-aminovinyl)-D-cysteine (S19-C22).
Literature Information
- ·Literature 1
-
Title
- Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings.
-
Pubmed ID
- 2835685
-
Reference
- Nature. 1988 May 19;333(6170):276-268.
-
Author
- Schnell N, Entian KD, Schneider U, Götz F, Zähner H, Kellner R, Jung G.
- ·Literature 2
-
Title
- Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic.
-
Pubmed ID
- 3769923
-
Reference
- Eur J Biochem. 1986 Oct 1;160(1):9-22.
-
Author
- Allgaier H, Jung G, Werner RG, Schneider U, Zähner H.