General Information
-
DRAMP ID
- DRAMP00062
-
Peptide Name
- Mersacidin (Bacteriocin; Preclinical)
-
Source
- Bacillus sp. HIL-Y85/54728 (Gram-positive bacteria)
-
Family
- Belongs to the type B lantibiotic family (Class I bacteriocin)
-
Gene
- mrsA
-
Sequence
- CTFTLPGGGGVCTLTSECIC
-
Sequence Length
- 20
-
UniProt Entry
- P43683
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+
-
Target Organism
-
- Gram-positive bacteria: Methicillin-resistant Staphylococcus aureus, Vancomycin-resistant Enterococcus, Clostridium difficile.
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- The sugar phosphate head group of the peptidoglycan precursorLipid II
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
Structure
- Non helix or strand structure (Turn)
-
Structure Description
- Not found
-
Helical Wheel Diagram
-
PDB ID
- 1QOW resolved by NMR.
-
Predicted Structure
- There is no predicted structure for DRAMP00062.
Physicochemical Information
-
Formula
- C81H132N20O28S4
Absent Amino Acids
- ADHKMNQRWY
Common Amino Acids
- CGT
Mass
- 1962.3
PI
- 4
Basic Residues
- 0
Acidic Residues
- 1
Hydrophobic Residues
- 5
Net Charge
- -1
-
Boman Index
- 10.17
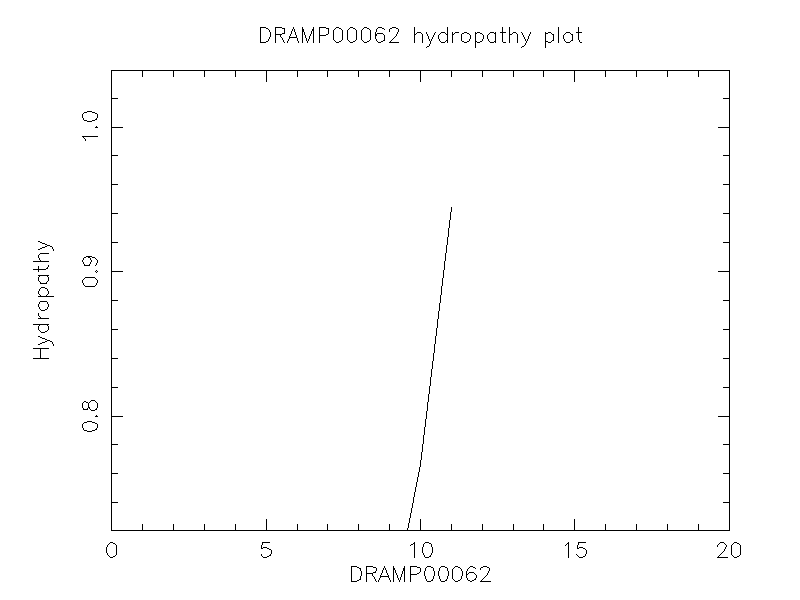
Hydrophobicity
- 0.94
Aliphatic Index
- 73
Half Life
-
- Mammalian:1.2 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 250
Absorbance 280nm
- 13.16
Polar Residues
- 13
DRAMP00062
Comments Information
Function
- Kills a number of Gram-positive bacteria. Acts at the level of cell wall biosynthesis by interfering with bacterial peptidoglycan biosynthesis. Specifically inhibits the conversion of the lipid II intermediate into polymeric nascent glycan strands by transglycosylation. May interact with the peptidoglycan precursor rather than with the enzyme.
PTM
- There are one dihydroalanine (Dha)
Literature Information
- ·Literature 1
-
Title
- Cloning, sequencing and production of the lantibiotic mersacidin.
-
Pubmed ID
- 7737474
-
Reference
- FEMS Microbiol Lett. 1995 Mar 15;127(1-2):121-126.
-
Author
- Bierbaum G, Brötz H, Koller KP, Sahl HG.
- ·Literature 2
-
Title
- Constitution and solution conformation of the antibiotic mersacidin determined by NMR and molecular dynamics.
-
Pubmed ID
- 9119018
-
Reference
- Eur J Biochem. 1997 Mar 1;244(2):501-512.
-
Author
- Prasch T, Naumann T, Markert RL, Sattler M, Schubert W, Schaal S, Bauch M, Kogler H, Griesinger C.
- ·Literature 3
-
Title
- Biosynthesis of the lantibiotic mersacidin: organization of a type B lantibiotic gene cluster.
-
Pubmed ID
- 10831439
-
Reference
- Appl Environ Microbiol. 2000 Jun;66(6):2565-2571.
-
Author
- Altena K, Guder A, Cramer C, Bierbaum G.
- ·Literature 4
-
Title
- Ab initio structure determination of the lantibiotic mersacidin.
-
Pubmed ID
- 10818347
-
Reference
- Acta Crystallogr D Biol Crystallogr. 2000 Jun;56(Pt 6):705-713.
-
Author
- Schneider TR, Kärcher J, Pohl E, Lubini P, Sheldrick GM.