General Information
-
DRAMP ID
- DRAMP00143
-
Peptide Name
- Plantaricin-A (PlnA; Bacteriocin)
-
Source
- Lactobacillus plantarum (strain ATCC BAA-793 / NCIMB 8826 / WCFS1) (Gram-positive bacteria)
-
Family
- Belongs to the class IIb bacteriocin
-
Gene
- plnA
-
Sequence
- KSSAYSLQMGATAIKQVKKLFKKWGW
-
Sequence Length
- 26
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial
-
Target Organism
- Closely related Lactobacillus
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Cell membrane
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
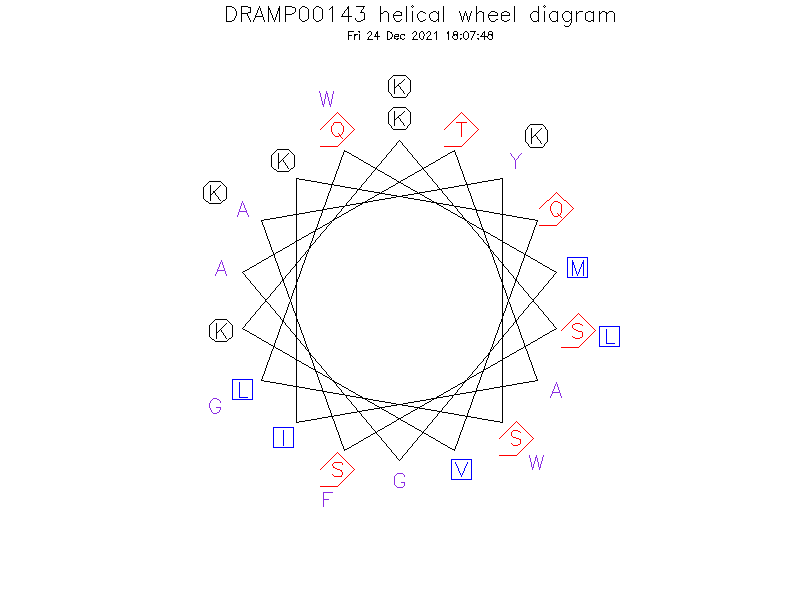
Structure
- Alpha helix
-
Structure Description
- Active plantaricin A is composed of an alpha chain and a beta chain. The sequence of alpha chain and beta chain, however, are essential identical with the only difference being one additional Ala at the N-terminus of chain b. The 3D structure of chain a in DPC micelles has been determined by NMR spectroscopy (J Biol Chem. 2005 Jun 17;280(24):22945-50).
-
Helical Wheel Diagram
-
PDB ID
- 1YTR resolved by NMR.
-
Predicted Structure
- There is no predicted structure for DRAMP00143.
Physicochemical Information
-
Formula
- C140H222N36O34S
Absent Amino Acids
- CDEHNPR
Common Amino Acids
- K
Mass
- 2985.58
PI
- 10.4
Basic Residues
- 6
Acidic Residues
- 0
Hydrophobic Residues
- 10
Net Charge
- +6
-
Boman Index
- -21.19
Hydrophobicity
- -0.423
Aliphatic Index
- 67.69
Half Life
-
- Mammalian:1.3 hour
- Yeast:3 min
- E.coli:2 min
Extinction Coefficient Cystines
- 12490
Absorbance 280nm
- 499.6
Polar Residues
- 7
DRAMP00143
Comments Information
Function
- This heat stable bacteriocin inhibits the growth of closely related Lactobacillus species. It may act as a pore-forming protein, creating a channel in the cell membrane through a "barrel stave" mechanism. Antibacterial activity requires both chain a (PlnA) and chain b (PlnB). The sequence of chain a and chain b, however, are essential identical with the only difference being one additional Ala at the N-terminus of chain b.
Subunit structure
- Active plantaricin A is composed of an alpha chain and a beta chain.
Miscellaneous
- The beta chain sequence is shown.
Literature Information
- ·Literature 1
-
Title
- Purification and characterization of plantaricin A, a Lactobacillus plantarum bacteriocin whose activity depends on the action of two peptides.
-
Pubmed ID
- 8245827
-
Reference
- J Gen Microbiol. 1993 Sep;139(9):1973-1978.
-
Author
- Nissen-Meyer J, Larsen AG, Sletten K, Daeschel M, Nes IF.