General Information
-
DRAMP ID
- DRAMP00162
-
Peptide Name
- Thuricin CDalpha (Trn-alpha; one peptide of Thuricin CD; Bacteriocin)
-
Source
- Bacillus thuringiensis DPC 6431 (Gram-positive bacteria)
-
Family
- Belongs to the class IIb bacteriocin
-
Gene
- Not found
-
Sequence
- GNAACVIGCIGSCVISEGIGSLVGTAFTLG
-
Sequence Length
- 30
-
UniProt Entry
- F5AT07
-
Protein Existence
- Predicted
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+
-
Target Organism
-
- Gram-positive bacteria: Clinical Clostridium difficile isolates (MIC in the range of nM) and Listeria monocytogenes, Lactobacillus fermentum.
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
Structure
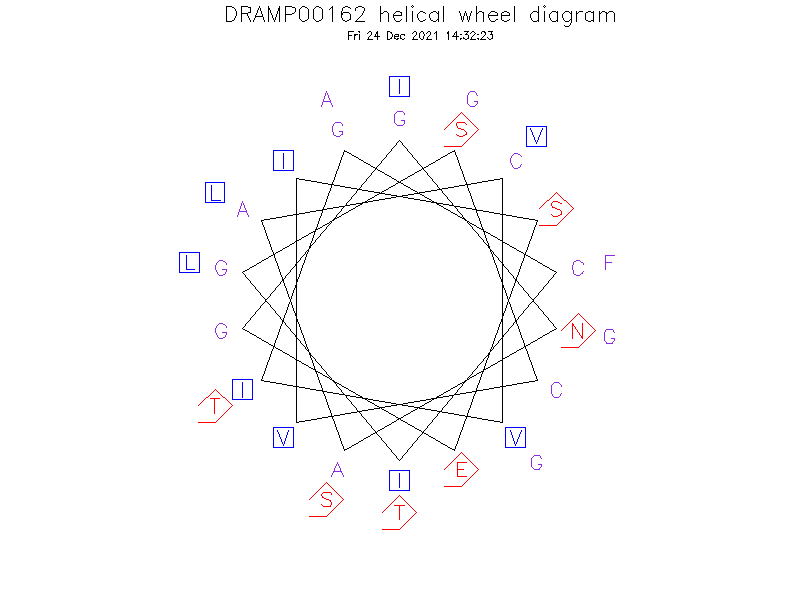
- Alpha helix (3 helices; 16 residues)
-
Structure Description
- Trn-α has L-stereochemistry at Ser21 (alpha-R), L-stereochemistry at Thr25 (alpha-R), and D-stereochemistry at Thr28 (alpha-S) (an LLD isomer).
-
Helical Wheel Diagram
-
PDB ID
- 2L9X resolved by NMR.
-
Predicted Structure
- There is no predicted structure for DRAMP00162.
Physicochemical Information
-
Formula
- C118H197N31O39S3
Absent Amino Acids
- DHKMPQRWY
Common Amino Acids
- G
Mass
- 2770.23
PI
- 4
Basic Residues
- 0
Acidic Residues
- 1
Hydrophobic Residues
- 13
Net Charge
- -1
-
Boman Index
- 31.68
Hydrophobicity
- 1.343
Aliphatic Index
- 117
Half Life
-
- Mammalian:30 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 125
Absorbance 280nm
- 4.31
Polar Residues
- 16
DRAMP00162
Comments Information
Function
- Thuricin CD is produced by Bacillus thuringiensis DPC 6431, a bacterial strain isolated from a human fecal sample, and it consists of two distinct peptides, Trn-alpha and Trn-beta, that act synergistically to kill a wide range of clinical C. difficile isolates. Thuricin CD showes no activity against any Gram-negative organisms tested, including Escherichia coli, Pseudomonas sp., Salmonella sp., and Bacteroides fragilis.
Biophysicochemical properties
- Cell-free supernatants of thuricin were active throughout the pH range of 2-9 and heat stable up to 85 °C. There was, however, a reduction in activity at 90 °C and a complete loss of activity at 100 °C after 15 min.
Literature Information
- ·Literature 1
-
Title
- Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile.
-
Pubmed ID
- 20435915
-
Reference
- Proc Natl Acad Sci U S A. 2010 May 18;107(20):9352-9357.
-
Author
- Rea MC, Sit CS, Clayton E, O'Connor PM, Whittal RM, Zheng J, Vederas JC, Ross RP, Hill C.
- ·Literature 2
-
Title
- The 3D structure of thuricin CD, a two-component bacteriocin with cysteine sulfur to alpha-carbon cross-links.
-
Pubmed ID
- 21526839
-
Reference
- J Am Chem Soc. 2011 May 25;133(20):7680-7683.
-
Author
- Sit CS, McKay RT, Hill C, Ross RP, Vederas JC.