General Information
-
DRAMP ID
- DRAMP00195
-
Peptide Name
- Colicin-V (Microcin-V; Bacteriocin)
-
Source
- Escherichia coli (Gram-negative bacteria)
-
Family
- Belongs to the class IIa microcin
-
Gene
- cvaC
-
Sequence
- ASGRDIAMAIGTLSGQFVAGGIGAAAGGVAGGAIYDYASTHKPNPAMSPSGLGGTIKQKPEGIPSEAWNYAAGRLCNWSPNNLSDVCL
-
Sequence Length
- 88
-
UniProt Entry
- P22522
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram-
-
Target Organism
-
- Gram-negative bacteria: Escherichia coli (also closely related bacteria), Enterobacteriaceae.
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Cell membrane
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
Structure
- Not found
-
Structure Description
- Not found
-
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP00195.
Physicochemical Information
-
Formula
- C381H594N108O120S4
Absent Amino Acids
- ?
Common Amino Acids
- GA
Mass
- 8735.8
PI
- 6.78
Basic Residues
- 6
Acidic Residues
- 5
Hydrophobic Residues
- 31
Net Charge
- +1
-
Boman Index
- -49.96
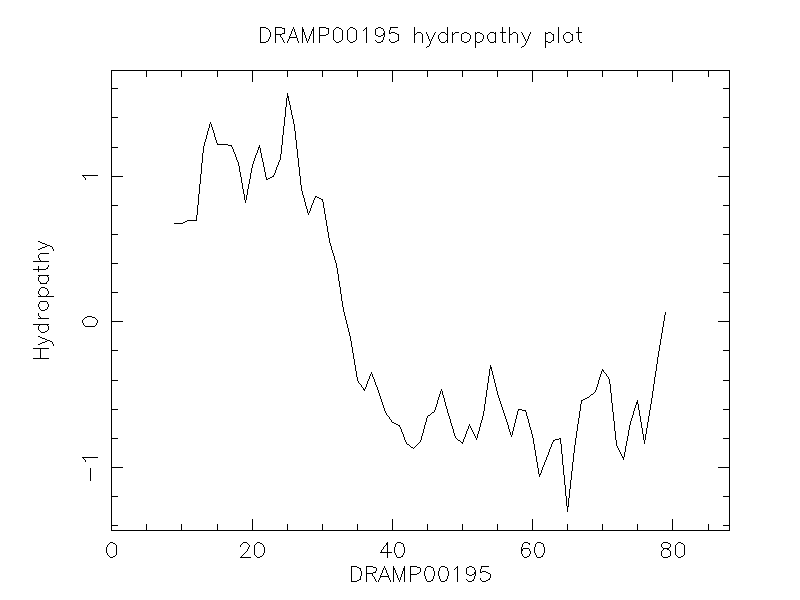
Hydrophobicity
- -0.003
Aliphatic Index
- 74.55
Half Life
-
- Mammalian:4.4 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 15595
Absorbance 280nm
- 179.25
Polar Residues
- 36
DRAMP00195
Comments Information
Function
- Colicin V kills sensitive cells by disrupting the membrane potential. It targets bacterial membranes and induces ion channel formation, leading to the disruption of the proton-motive force and hence ATP production.
PTM
- Contains one disulfide bond between 76-87.
Literature Information
- ·Literature 1
-
Title
- Purification and characterization of colicin V from Escherichia coli culture supernatants.
-
Pubmed ID
- 8204625
-
Reference
- Biochemistry. 1994 Jun 7;33(22):6911-6917.
-
Author
- Fath MJ, Zhang LH, Rush J, Kolter R.
- ·Literature 2
-
Title
- The leader peptide of colicin V shares consensus sequences with leader peptides that are common among peptide bacteriocins produced by Gram-positive bacteria.
-
Pubmed ID
- 7952189
-
Reference
- Microbiology. 1994 Sep;140 (Pt 9):2383-2389.
-
Author
- H¥varstein LS, Holo H, Nes IF.
- ·Literature 3
-
Title
- Genetic analysis of an MDR-like export system: the secretion of colicin V.
-
Pubmed ID
- 2249654
-
Reference
- EMBO J. 1990 Dec;9(12):3875-3884.
-
Author
- Gilson L, Mahanty HK, Kolter R.