General Information
-
DRAMP ID
- DRAMP01095
-
Peptide Name
- Bombinin-like peptide 2 (Contains: Bombinin H2; toads, amphibians, animals)
-
Source
- Bombina variegata (Yellow-bellied toad)
-
Family
- Belongs to the bombinin family
-
Gene
- Not found
-
Sequence
- GIGASILSAGKSALKGFAKGLAEHFAN
-
Sequence Length
- 27
-
UniProt Entry
- P82286
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial
-
Target Organism
- No MICs found in DRAMP database
-
Hemolytic Activity
-
- [Ref:10333736]Non-hemolytic activity
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
Structure
- Not found
-
Structure Description
- Not found
-
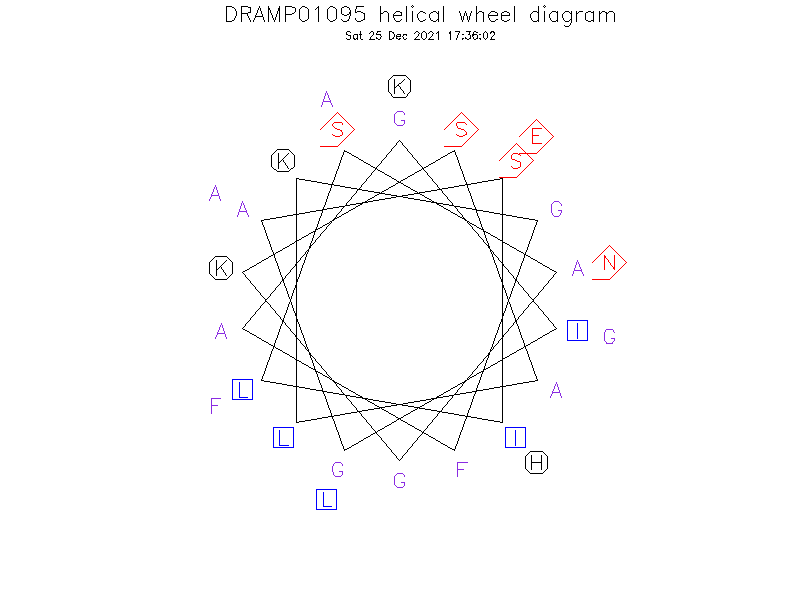
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP01095.
Physicochemical Information
-
Formula
- C118H191N33O34
Absent Amino Acids
- CDMPQRTVWY
Common Amino Acids
- A
Mass
- 2616.02
PI
- 9.7
Basic Residues
- 4
Acidic Residues
- 1
Hydrophobic Residues
- 13
Net Charge
- +3
-
Boman Index
- 1.16
Hydrophobicity
- 0.389
Aliphatic Index
- 94.44
Half Life
-
- Mammalian:30 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 9
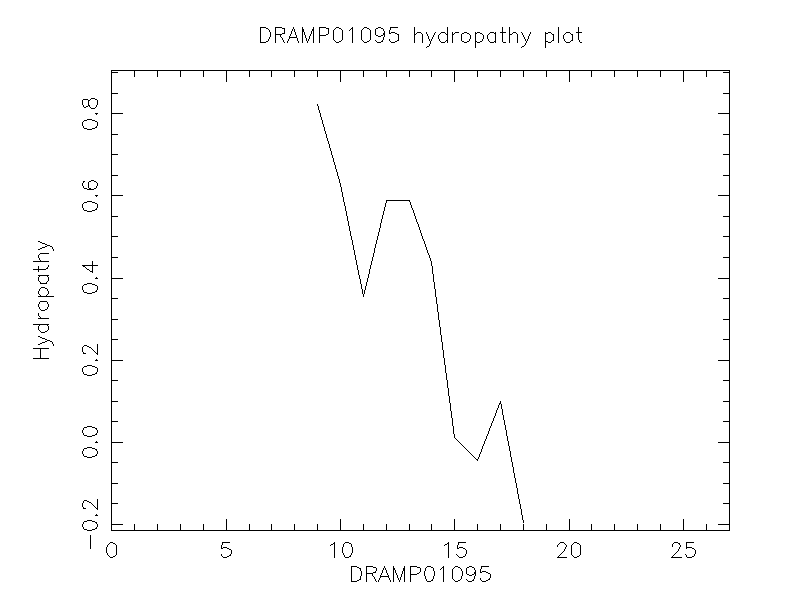
DRAMP01095
Comments Information
Function
- Bombinin-like peptide 2 has antimicrobial activity, but no hemolytic activity. Preliminary evidence indicates that this peptide does not lyse and thus kill the bacteria by its antimicrobial activity. Bombinin H2 has antibacterial and hemolytic activity.
Tissue specificity
- Expressed by the skin glands.
Literature Information
- ·Literature 1
-
Title
- Antimicrobial peptides from amphibian skin: what do they tell us?
-
Pubmed ID
- 10333736
-
Reference
- Biopolymers. 1998;47(6):435-450.
-
Author
- Simmaco M, Mignogna G, Barra D.
- ·Literature 2
-
Title
- Antibacterial and haemolytic peptides containing D-alloisoleucine from the skin of Bombina variegata.
-
Pubmed ID
- 223491
-
Reference
- EMBO J. 1993 Dec;12(12):4829-4832.
-
Author
- Mignogna G, Simmaco M, Kreil G, Barra D.