General Information
-
DRAMP ID
- DRAMP01164
-
Peptide Name
- Bombinin (toads, amphibians, animals)
-
Source
- Bombina variegata (Yellow-bellied toad)
-
Family
- Not found
-
Gene
- Not found
-
Sequence
- GIGALSAKGALKGLAKGLAEHFAN
-
Sequence Length
- 24
-
UniProt Entry
- P01505
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-
-
Target Organism
-
- [Ref.8223491]Gram-negative bacterium: Escherichia coli D21 (MIC=3 µM);
- Gram-positive bacterium: Staphylococcus aureus Cowan 1 (MIC=14 µM).
-
Hemolytic Activity
-
- [Ref.8223491] The lethal concentration (LC, the lowest concentration that inhibits growth) was over 50 μM
-
Cytotoxicity
- No cytotoxicity information found in the reference(s) presented
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Amidation
-
Nonterminal Modifications and Unusual Amino Acids
- None
-
Stereochemistry
- L
-
Structure
- Not found
-
Structure Description
- Not found
-
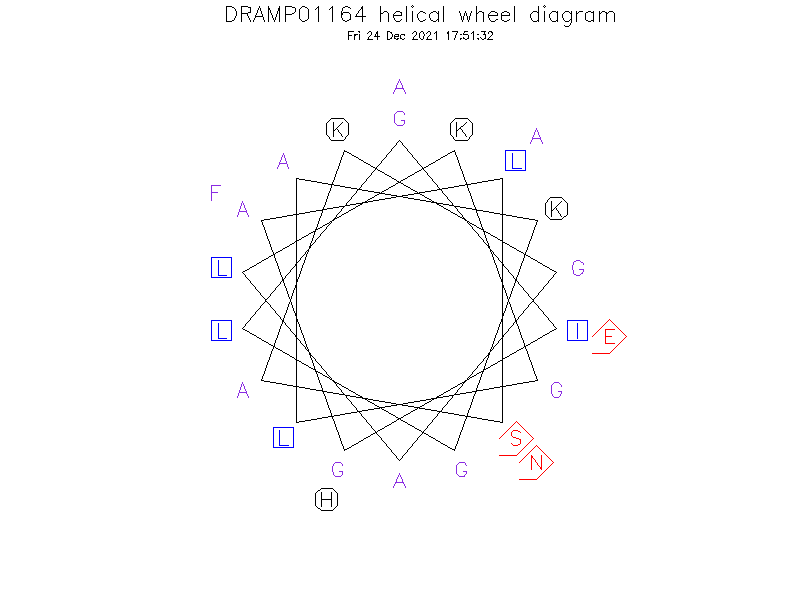
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP01164.
Physicochemical Information
-
Formula
- C103H172N30O29
Absent Amino Acids
- CDMPQRTVWY
Common Amino Acids
- A
Mass
- 2294.68
PI
- 9.7
Basic Residues
- 4
Acidic Residues
- 1
Hydrophobic Residues
- 12
Net Charge
- +3
-
Boman Index
- 4.98
Hydrophobicity
- 0.358
Aliphatic Index
- 106.25
Half Life
-
- Mammalian:30 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 7
DRAMP01164
Comments Information
Function
- Has antimicrobial and hemolytic activities.
Tissue specificity
- Expressed by the skin glands.
PTM
- C-terminal amidation.
Caution
- Residue 20 is assigned as E by similarity to other bombin sequences instead of Z (Glutamic acid or Gutamine) given
Literature Information
- ·Literature 1
-
Title
- Isolation and structural resolution of a haemolytically active polypeptide from the immune secretion of a European toad.
-
Pubmed ID
- PubMed ID is not available
-
Reference
- Monatsh. Chem. 1970;101:182-189.
-
Author
- Csordas A, Michl H.
- ·Literature 2
-
Title
- Antibacterial and haemolytic peptides containing D-alloisoleucine from the skin of Bombina variegata.
-
Pubmed ID
- 8223491
-
Reference
- EMBO J. 1993; 12:4829-4832.
-
Author
- Mignogna G, Simmaco M, Kreil G, Barra D.