General Information
-
DRAMP ID
- DRAMP01214
-
Peptide Name
- Kassinatuerin-2Ma (Frogs, amphibians, animals)
-
Source
- Kassina maculata (African hyperoliid frog)
-
Family
- Not found
-
Gene
- Not found
-
Sequence
- FLGAIAAALPHVINAVTNAL
-
Sequence Length
- 20
-
UniProt Entry
- No entry found
-
Protein Existence
- Not found
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-
-
Target Organism
-
- [Ref.19427345]Gram-positive bacterium: Staphylococcus aureus (NCTC 10788) (MIC=16 µM);
- Gram-negative bacterium: Escherichia coli (NCTC 10418) (MIC>200 µM) (MIC=120 µM);
- Yeast: Candida albicans (NCPF 1467) (MIC=120 µM).
-
Hemolytic Activity
-
- [Ref:19427345]18% hemolytic activity at 16 μM, 45–50% hemolytic activity at 120 μM against horse erythrocytes
-
Cytotoxicity
- No cytotoxicity information found in the reference(s) presented
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Amidation
-
Nonterminal Modifications and Unusual Amino Acids
- Free
-
Stereochemistry
- L
-
Structure
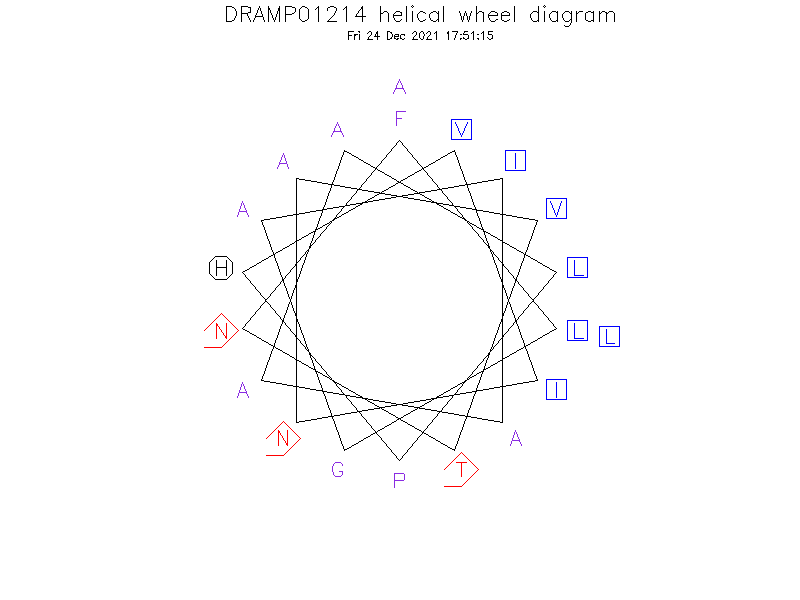
- Alpha helix
-
Structure Description
- Secondary structure prediction analysis revealed that virtually the entire structure of each kassinatuerin-2 related peptide is composed of alpha-helix, the helix was not amphipathic like many other amphibian skin antimicrobial peptides, in which there is a hydrophobic face and a positively charged faces the latter presumably interacting with the negatively charged glycocalyx of cell membranes.
-
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP01214.
Physicochemical Information
-
Formula
- C92H150N24O24
Absent Amino Acids
- CDEKMQRSWY
Common Amino Acids
- A
Mass
- 1976.35
PI
- 6.74
Basic Residues
- 1
Acidic Residues
- 0
Hydrophobic Residues
- 14
Net Charge
- +1
-
Boman Index
- 26.95
Hydrophobicity
- 1.475
Aliphatic Index
- 156.5
Half Life
-
- Mammalian:1.1 hour
- Yeast:3 min
- E.coli:2 min
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 4
DRAMP01214
Comments Information
Function
- Natural kassinatuerin-2Ma and their synthetic replicates possessed relatively potent growth inhibitory activity against the Gram-positive bacterium, S. aureus, but were ineffective against the Gram-negative bacterium, E. coli at concentrations up to 200 µM and were relatively ineffective against the pathogenic yeast, C. albicans (120 µM). Also possessed hemolytic activity as demonstrated using horse erythrocytes.
Literature Information
- ·Literature 1
-
Title
- A family of kassinatuerin-2 related peptides from the skin secretion of the African hyperoliid frog, Kassina maculata.
-
Pubmed ID
- 19427345
-
Reference
- Peptides. 2009 Aug;30(8):1428-1433.
-
Author
- Wang L, Zhou M, McGrath S, Chen T, Gorman SP, Walker B, Shaw C.