General Information
-
DRAMP ID
- DRAMP01555
-
Peptide Name
- Caerin-1.5 (Frogs, amphibians, animals)
-
Source
- Litoria caerulea (Green tree frog)
-
Family
- Belongs to the frog skin active peptide family (Caerin subfamily)
-
Gene
- Not found
-
Sequence
- GLLSVLGSVVKHVIPHVVPVIAEHL
-
Sequence Length
- 25
-
UniProt Entry
- P56230
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-,Antiviral
-
Target Organism
-
- [Ref.15203252]Gram-positive bacteria: Bacillus cereus (MIC=50 µg/ml), Leuconostoc lactis (MIC=3 µg/ml), Listeria innocua (MIC=50 µg/ml), Micrococcus luteus (MIC=12 µg/ml), Staphylococcus aureus (MIC=25 µg/ml), Staphylococcus epidermis (MIC=25 µg/ml), Streptococcus uberis (MIC=50 µg/ml);
- Gram-negative bacterium: Pasteurella multocida (MIC=25 µg/ml).
- [Ref.26026377]Virus:HIV: inhibition of HIV Pseudovirus (PsV) infection in CD4+ T cells(IC50=3 µM).
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- [Ref.26026377]Human endocervical cells End1/E6E7:50% cell death at 12.5 µM.
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Amidation
-
Nonterminal Modifications and Unusual Amino Acids
- Free
-
Stereochemistry
- L
-
Structure
- Not found
-
Structure Description
- Not found
-
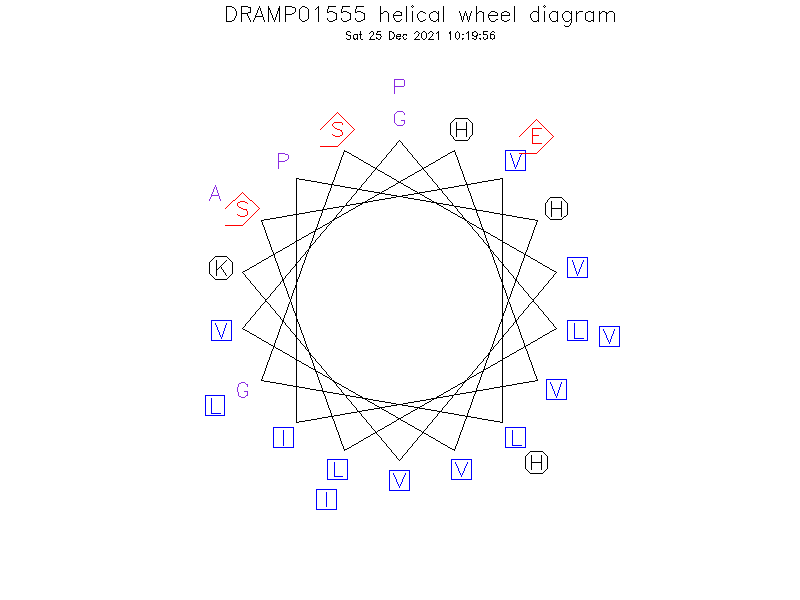
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP01555.
Physicochemical Information
-
Formula
- C123H206N32O30
Absent Amino Acids
- CDFMNQRTWY
Common Amino Acids
- V
Mass
- 2613.19
PI
- 7.02
Basic Residues
- 4
Acidic Residues
- 1
Hydrophobic Residues
- 14
Net Charge
- +3
-
Boman Index
- 28.35
Hydrophobicity
- 1.312
Aliphatic Index
- 178.8
Half Life
-
- Mammalian:30 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 4
DRAMP01555
Comments Information
Function
- Antibacterial peptide, that adopts an alpha helical conformation which can disrupt bacterial membranes. Each caerin displays a different antimicrobial specificity.
Tissue specificity
- Expressed by the skin parotoid and/or rostral glands.
Domain
- Contains two amphipathic alpha helix regions separated by a region of less-defined helicity and greater flexibility (By similarity).
PTM
- C-terminal amidation.
Literature Information
- ·Literature 1
-
Title
- Inhibition of HIV infection by caerin 1 antimicrobial peptides.
-
Pubmed ID
- 26026377
-
Reference
- Peptides. 2015 Sep;71:296-303.
-
Author
- VanCompernolle S, Smith PB, Bowie JH, Tyler MJ, Unutmaz D, Rollins-Smith LA.
- ·Literature 2
-
Title
- Host-defence peptides of Australian anurans: structure, mechanism of action and evolutionary significance.
-
Pubmed ID
- 15203252
-
Reference
- Peptides. 2004 Jun;25(6):1035-1054.
-
Author
- Apponyi MA, Pukala TL, Brinkworth CS, Maselli VM, Bowie JH, Tyler MJ, Booker GW, Wallace JC, Carver JA, Separovic F, Doyle J, Llewellyn LE.
- ·Literature 3
-
Title
- Peptides from Australian frogs. The structures of the caerins from Litoria caerula.
-
Pubmed ID
- PubMed ID is not available
-
Reference
- J. Chem. Res. 1993; 138: 910-936.
-
Author
- Stone DJM, Waugh RJ, Bowie JH, Wallace JC, Tyler MJ.