General Information
-
DRAMP ID
- DRAMP01649
-
Peptide Name
- Dermaseptin-BI (Dermaseptin B1; Frogs, amphibians, animals)
-
Source
- Phyllomedusa bicolor (Two-colored leaf frog) (Rana bicolor)
-
Family
- Belongs to the frog skin active peptide family (Dermaseptin subfamily)
-
Gene
- Not found
-
Sequence
- AMWKDVLKKIGTVALHAGKAALGAVADTISQ
-
Sequence Length
- 31
-
UniProt Entry
- P80282
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-, Antifungal
-
Target Organism
-
- Gram-negative bacterium: Escherichia coli (IP 76-24) (MIC=15 µg/ml);
- Gram-positive bacterium: Nocardia brasiliensis (IP 16-80) (MIC=30 µg/ml).
- Fungi: Tricophyton rubrum (IP 1400-82) (MIC=15 µg/ml), Microsporum canis (IP 1194) (MIC=10µg/ml), Arthroderma simii (IP 1063-74) (MIC=30 µg/ml), Cryptococcus neoformans (IP 960-67) (MIC=15 µg/ml), Cryptococcus neofonnans (IP 962-67) (MIC=15 µg/ml), Aspergillus fumigatus (IP 1025-70)(MIC=125 µg/ml), Aerornonas caviae (IP 67-16 T) (MIC=60 µg/ml), Candida albicans (IP 884-65) (MIC=15 µg/ml).
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
- No cytotoxicity information found in the reference(s) presented
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Amidation
-
Nonterminal Modifications and Unusual Amino Acids
- Free
-
Stereochemistry
- L
-
Structure
- Not found
-
Structure Description
- Not found
-
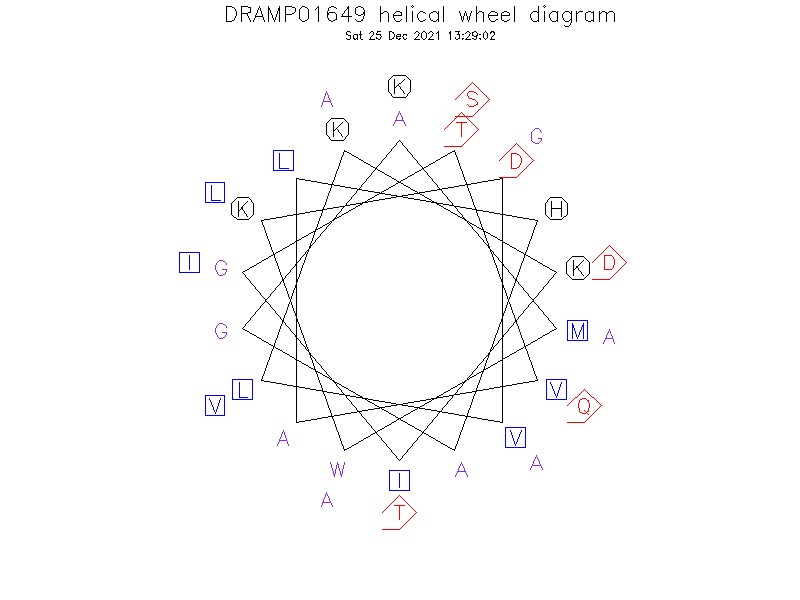
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP01649.
Physicochemical Information
-
Formula
- C142H239N39O40S
Absent Amino Acids
- CEFNPRY
Common Amino Acids
- A
Mass
- 3164.76
PI
- 9.53
Basic Residues
- 5
Acidic Residues
- 2
Hydrophobic Residues
- 16
Net Charge
- +3
-
Boman Index
- -1.49
Hydrophobicity
- 0.448
Aliphatic Index
- 113.55
Half Life
-
- Mammalian:4.4 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 5500
Absorbance 280nm
- 183.33
Polar Residues
- 6
DRAMP01649
Comments Information
Function
- Possesses a potent antimicrobial activity against bacteria, fungi and protozoa. Probably acts by disturbing membrane functions with its amphipathic structure.
Tissue specificity
- Expressed by the skin glands.
PTM
- C-terminal amidation (Potential).
Literature Information
- ·Literature 1
-
Title
- Isolation and structure of novel defensive peptides from frog skin.
-
Pubmed ID
- 8306981
-
Reference
- Eur J Biochem. 1994 Jan 15;219(1-2):145-154.
-
Author
- Mor A, Nicolas P.
- ·Literature 2
-
Title
- Precursors of vertebrate peptide antibiotics dermaseptin b and adenoregulin have extensive sequence identities with precursors of opioid peptides dermorphin, dermenkephalin, and deltorphins.
-
Pubmed ID
- 8074751
-
Reference
- J Biol Chem. 1994 Jul 8;269(27):17847-17852.
-
Author
- Amiche M, Ducancel F, Mor A, Boulain JC, Menez A, Nicolas P.