General Information
-
DRAMP ID
- DRAMP02136
-
Peptide Name
- Antimicrobial peptide 7 (XT-7; Frogs, amphibians, animals)
-
Source
- Xenopus tropicalis (Western clawed frog) (Silurana tropicalis)
-
Family
- Not found
-
Gene
- Not found
-
Sequence
- GLLGPLLKIAAKVGSNLL
-
Sequence Length
- 18
-
UniProt Entry
- P84381
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-, Antifungal
-
Target Organism
-
- Gram-positive bacterium: Staphylococcus aureus (MIC=5 µM);
- Gram-negative bacterium: Escherichia coli (MIC=5 µM);
- yeast Candida albicans (MIC=40 µM).
- Clinical isolates: Staphylococcus aureus 8325 (MIC=6 µM), Staphylococcus aureus (MRSA) (MIC=7 µM), Staphylococcus epidermidis 1420129 (MIC=3 µM), S. saprophyticus 1950556 (MIC=13 µM), Escherichia coli 25922 (MIC=6 µM), Streptococcus group C 1541035 (MIC=3 µM), Shigella sonnei 1840187 (MIC=35 µM), Pseudomonas aeruginosa 0380972 (MIC=60 µM), Enterobacter cloacae 1441323 (MIC=35 µM).
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Cell membrane
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
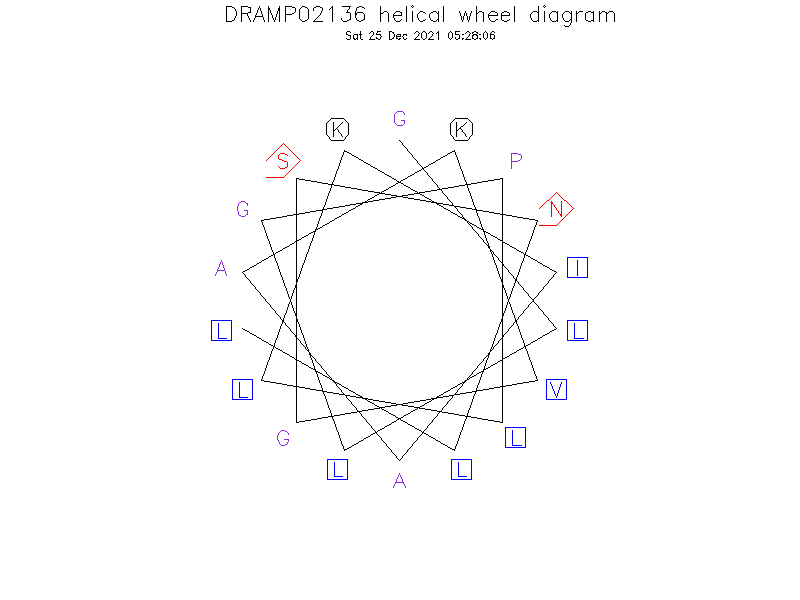
Structure
- Alpha helix
-
Structure Description
- Not found
-
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP02136.
Physicochemical Information
-
Formula
- C83H149N21O21
Absent Amino Acids
- CDEFHMQRTWY
Common Amino Acids
- L
Mass
- 1777.22
PI
- 10
Basic Residues
- 2
Acidic Residues
- 0
Hydrophobic Residues
- 10
Net Charge
- +2
-
Boman Index
- 23.78
Hydrophobicity
- 1.122
Aliphatic Index
- 178.89
Half Life
-
- Mammalian:30 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 5
DRAMP02136
Comments Information
Function
- Has very strong antibacterial activity against many Gram-negative bacteria and the Gram-positive bacteria S.pneumoniae and Enterococcus sp. There is moderate antimicrobial activity against the Gram-negative bacterium P.aeruginosa and yeast C. albicans and weaker activity against the Streptococcal strains and E.coli. Has hemolytic activity against human red blood cells (HC50=70 µM). Seems to disrupt the membranes by adopting an alpha-helical conformation.
Tissue specificity
- Expressed by the skin glands.
PTM
- Amidation on Leu-18 is required to adopt an alpha-helical conformation in a hydrophobic solvent. The absence of amidation leads to a decrease in cationicity leading to a more than a 10-fold decrease in antimicrobial activities.
Literature Information
- ·Literature 1
-
Title
- Antimicrobial peptides isolated from skin secretions of the diploid frog, Xenopus tropicalis (Pipidae).
-
Pubmed ID
- 11738090
-
Reference
- Biochim Biophys Acta. 2001 Nov 26;1550(1):81-89.
-
Author
- Ali MF, Soto A, Knoop FC, Conlon JM.