General Information
-
DRAMP ID
- DRAMP02271
-
Peptide Name
- Magainin-2 (Magainin II; chain of Magainins; Frogs, amphibians, animals)
-
Source
- Xenopus ruwenzoriensis (Uganda clawed frog)
-
Family
- Belongs to the gastrin/cholecystokinin family. Magainin subfamily.
-
Gene
- N/A
-
Sequence
- GIGKFLHSAKKFGKAFVGEIMNS
-
Sequence Length
- 23
-
UniProt Entry
- C0HKN6
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-, Antifungal, Antiprotozoal
-
Target Organism
-
- Gram-negative bacteria: Escherichia coli D31 (MIC=5 µg/ml), Klebsiella pneumoniae (MIC=10 µg/ml), Escherichia coli (MIC=50 µg/ml), Pseudomonas putida (MIC=10 µg/ml), Citrobacter freundii (MIC=30 µg/ml), Enterobacter cloacae (MIC=50 µg/ml), Pseudomonas aeruginosa (MIC=100 µg/ml), Serratia marcescens (MIC=100 µg/ml), Proteus mirabilis (MIC>100 µg/ml);
- Gram-positive bacteria: Staphylococcus epidermidis (MIC=10 µg/ml), Staphylococcus aureus (MIC=50 µg/ml), Streptococcus fecalis (MIC>100 µg/ml).
- Protozoa: Paramecium caudatum (MIC=10 µg/ml).
- Yeast: Candida albicans (MIC=80 µg/ml).
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
Structure
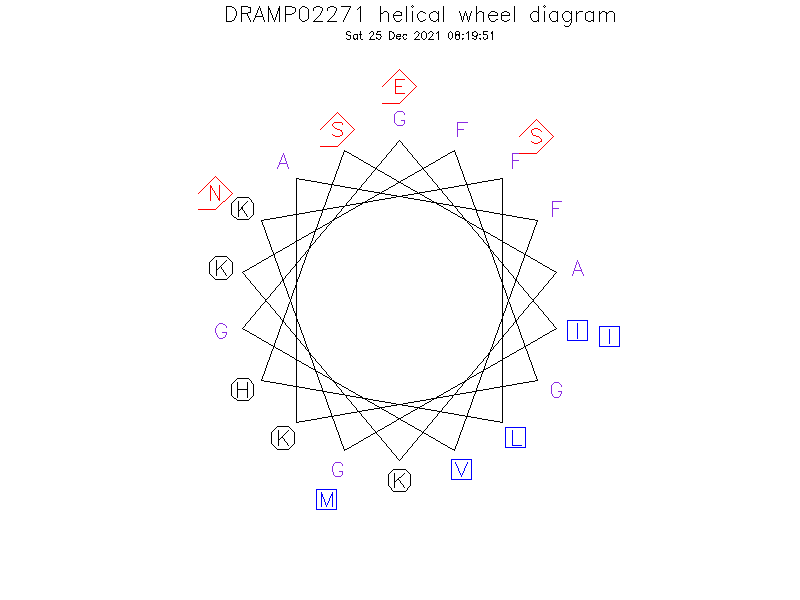
- Alpha helix
-
Structure Description
- The secondary structure is shown to be helical in dodecylphosphocholine micelles, sodium dodecylsulfate micelles, and trifluoroethanol/water solution.
-
Helical Wheel Diagram
-
Predicted Structure
- There is no predicted structure for DRAMP02271.
Physicochemical Information
-
Formula
- C114H180N30O29S
Absent Amino Acids
- CDPQRTWY
Common Amino Acids
- GK
Mass
- 2466.93
PI
- 10
Basic Residues
- 5
Acidic Residues
- 1
Hydrophobic Residues
- 9
Net Charge
- +4
-
Boman Index
- -9.64
Hydrophobicity
- 0.083
Aliphatic Index
- 72.17
Half Life
-
- Mammalian:30 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 7
DRAMP02271
Comments Information
Function
- Antimicrobial peptides that inhibit the growth of numerous species of bacteria and fungi and induce osmotic lysis of protozoa. Magainins are membrane lytic agents.
Tissue specificity
- Synthesized in the stomach and stored in a novel granular multinucleated cell in the gastric mucosa. It is stored as active, processed peptides in large granules within the granular gland secretions of the skin.
Literature Information
- ·Literature 1
-
Title
- Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor.
-
Pubmed ID
- 3299384
-
Reference
- Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449-5453.
-
Author
- Zasloff M.
- ·Literature 2
-
Title
- Two-dimensional 1H NMR experiments show that the 23-residue magainin antibiotic peptide is an alpha-helix in dodecylphosphocholine micelles, sodium dodecylsulfate micelles, and trifluoroethanol/water solution.
-
Pubmed ID
- 9090128
-
Reference
- J Biomol NMR. 1997 Feb;9(2):127-135.
-
Author
- Gesell J, Zasloff M, Opella SJ.
- ·Literature 3
-
Title
- All Atom Simulations of the Initial Binding of Magainin and Pleurocidin to Membranes Comprising a Mixture of Anionic and Zwitterionic
-
Pubmed ID
- PubMed ID is not available
-
Reference
- To be Published.
-
Author
- Vermeer LS, Kozlowska J, Lorenz CD, Mason JA.