General Information
-
DRAMP ID
- DRAMP02853
-
Peptide Name
- Cathelicidin-3 (Bactenecin-7, Bac7; PR-59; mammals, animals)
-
Source
- Bos taurus (Bovine)
-
Family
- Belongs to the cathelicidin family
-
Gene
- CATHL3
-
Sequence
- RRIRPRPPRLPRPRPRPLPFPRPGPRPIPRPLPFPRPGPRPIPRPLPFPRPGPRPIPRP
-
Sequence Length
- 59
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram-
-
Target Organism
-
- Gram-negative bacterium: Escherichia coli.
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
Structure
- Not found
-
Structure Description
- Not found
-
Helical Wheel Diagram
-
PDB ID
- 5HAU
-
Predicted Structure
- There is no predicted structure for DRAMP02853.
Physicochemical Information
-
Formula
- C323H526N110O60
Absent Amino Acids
- ACDEHKMNQSTVWY
Common Amino Acids
- P
Mass
- 6910.43
PI
- 13.2
Basic Residues
- 17
Acidic Residues
- 0
Hydrophobic Residues
- 11
Net Charge
- +17
-
Boman Index
- -202.52
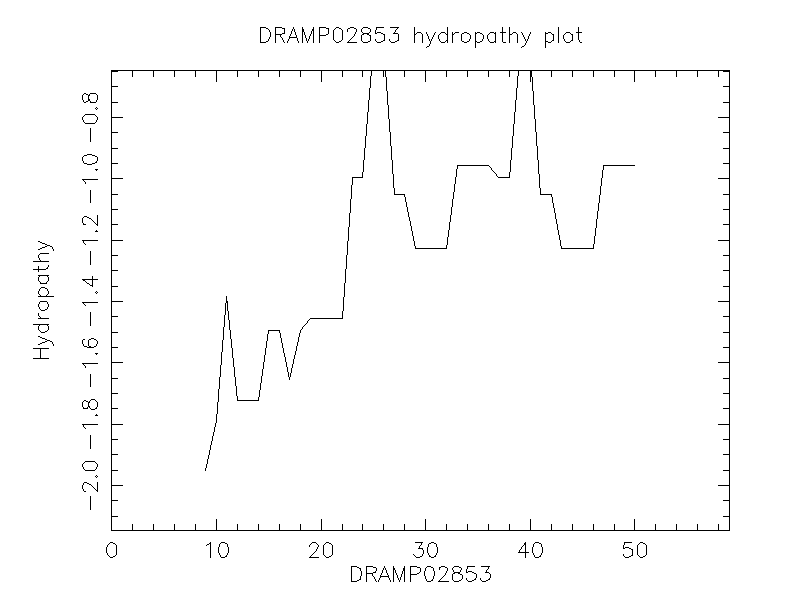
Hydrophobicity
- -1.371
Aliphatic Index
- 52.88
Half Life
-
- Mammalian:1 hour
- Yeast:2 min
- E.coli:2 min
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 3
DRAMP02853
Comments Information
Function
- Exerts, in vitro, a potent antimicrobial activity. Probably due to an impairment of the function of the respiratory chain and of energy-dependent activities in the inner membrane of susceptible microorganisms.
Tissue specificity
- Large granules of neutrophils.
PTM
- Contains two disulfide bonds.
Literature Information
- ·Literature 1
-
Title
- Amino acid sequences of two proline-rich bactenecins. Antimicrobial peptides of bovine neutrophils.
-
Pubmed ID
- 2229048
-
Reference
- J Biol Chem. 1990 Nov 5;265(31):18871-18874.
-
Author
- Frank RW, Gennaro R, Schneider K, Przybylski M, Romeo D.