General Information
-
DRAMP ID
- DRAMP02933
-
Peptide Name
- Polyphemusin-1 (PM1; crabs, Arthropods, animals)
-
Source
- Limulus polyphemus (Atlantic horseshoe crab)
-
Family
- Belongs to the tachyplesin/polyphemusin family
-
Gene
- Not found
-
Sequence
- RRWCFRVCYRGFCYRKCR
-
Sequence Length
- 18
-
UniProt Entry
- P14215
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-, Antifungal
-
Target Organism
-
- Gram-negative bacteria: Escherichia coli UB1005 (MIC=0.5 µg/ml), E. coli DC2 (MIC=1 µg/ml), Salmonella typhimurium (defensin-sensitive) (MIC=0.25 µg/ml), S. typhimurium (MIC=1 µg/ml), Pseudomonas aeruginosa K799 (MIC=2 µg/ml), P. aeruginosa Z61 (MIC=1 µg/ml);
- Gram-positive bacteria: Staphylococcus aureus SAP0017 (MIC=2 µg/ml), Staphylococcus epidermidis (MIC=1 µg/ml), Enterococcus faecalis (MIC=1 µg/ml).
- Yeasy: Candida albicans (MIC=4 µg/ml).
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
- No cytotoxicity information found in the reference(s) presented
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Cyclic
-
N-terminal Modification
- Free
-
C-terminal Modification
- Amidation
-
Nonterminal Modifications and Unusual Amino Acids
- Disulfide bond between Cys4 and Cys17,Cys8 and Cys13.
-
Stereochemistry
- L
-
Structure
- Beta strand (2 strands; 4 residues)
-
Structure Description
- The solution structure of polyphemusin I is determined using (1)H-NMR spectroscopy. Polyphemusin I is found to be an amphipathic, beta-hairpin connected by a type I' beta-turn.
-
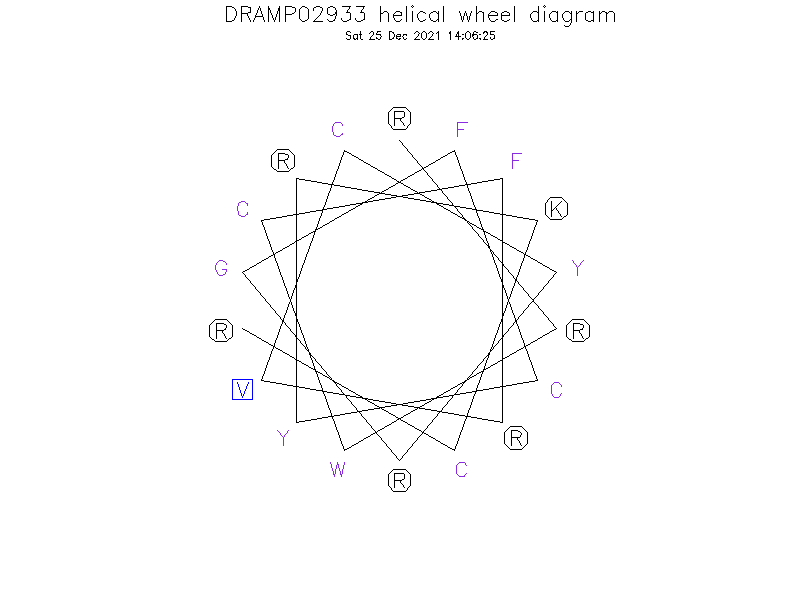
Helical Wheel Diagram
-
PDB ID
- 1RKK resolved by NMR.
-
Predicted Structure
- There is no predicted structure for DRAMP02933.
Physicochemical Information
-
Formula
- C108H164N38O21S4
Absent Amino Acids
- ADEHILMNPQST
Common Amino Acids
- R
Mass
- 2458.97
PI
- 10.33
Basic Residues
- 7
Acidic Residues
- 0
Hydrophobic Residues
- 4
Net Charge
- +7
-
Boman Index
- -76.96
Hydrophobicity
- -0.833
Aliphatic Index
- 16.11
Half Life
-
- Mammalian:1 hour
- Yeast:2 min
- E.coli:2 min
Extinction Coefficient Cystines
- 8730
Absorbance 280nm
- 513.53
Polar Residues
- 7
DRAMP02933
Comments Information
Function
- PM1 showes high antimicrobial activity against the Gram-negative, Gram-positive and fungal specimens tested.
Tissue specificity
- Hemocytes.
PTM
- Contains two disulfide bonds 4-17; 8-13 and C-terminal amidation.
Literature Information
- ·Literature 1
-
Title
- Antimicrobial peptides, isolated from horseshoe crab hemocytes, tachyplesin II, and polyphemusins I and II: chemical structures and biological activity.
-
Pubmed ID
- 2514185
-
Reference
- J Biochem. 1989 Oct;106(4):663-668.
-
Author
- Miyata T, Tokunaga F, Yonega T, Yoshikawa K, Iwanaga S, Niwa M, Takao T, Shimonishi Y.
- ·Literature 2
-
Title
- Structure-activity relationships for the beta-hairpin cationic antimicrobial peptide polyphemusin I.
-
Pubmed ID
- 15134657
-
Reference
- Biochim Biophys Acta. 2004 May 6;1698(2):239-250.
-
Author
- Powers JP, Rozek A, Hancock RE.