General Information
-
DRAMP ID
- DRAMP03405
-
Peptide Name
- mCRAMP-1 (mouse cathelin-related antimicrobial peptide 1; cathelicidin; Rodents, mammals, animals
-
Source
- Mus musculus (Mouse)
-
Family
- Not found
-
Gene
- Camp
-
Sequence
- GLLRKGGEKIGEKLKKIGQKIKNFFQKLVPQPE
-
Sequence Length
- 33
-
Protein Existence
- Transcript level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-, Antifungal
-
Target Organism
-
- Gram-negative bacteria: Escherichia coli ATCC 25922 (MIC=1 µM), Escherichia coli ML35 (MIC=2 µM), Escherichia coli D21 (MIC=8 µM), Pseudomonas aeruginosa ATCC 27853 (MIC=4 µM), Serratia marcescens ATCC 8100 (MIC=4 µM);
- Gram-positive bacteria: Staphylococcus aureus ATCC 25923 (MIC=32 µM), Staphylococcus aureus Cowan I (MIC=32 µM), Staphylococcus aureus (MRSA) (MIC>64 µM), Staphylococcus aureus (MRSA) (MIC=64 µM), Staphylococcus epidermidis ATCC 12228 (MIC=16 µM), Streptococcus faecalis ATCC 29212 (MIC=16 µM), Bacillus megaterium Bm11 (MIC=4 µM).
- Fungi: Candida albicans (MIC>64 µM), Cryptococcus neoformans (MIC=16 µM).
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Free
-
Nonterminal Modifications and Unusual Amino Acids
- Free
-
Stereochemistry
- L
-
Structure
- Alpha helix (CD)
-
Structure Description
- The spectra of 10-25 µm synthetic CRAMP-1 in buffer suggest that the peptide is in a random coil configuration and that it will assume a helical structure in the presence of 15–45% (v/v) trifluoroethanol.
-
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP03405.
Physicochemical Information
-
Formula
- C173H294N48O44
Absent Amino Acids
- ACDHMSTWY
Common Amino Acids
- K
Mass
- 3750.53
PI
- 10.22
Basic Residues
- 9
Acidic Residues
- 3
Hydrophobic Residues
- 10
Net Charge
- +6
-
Boman Index
- -53.87
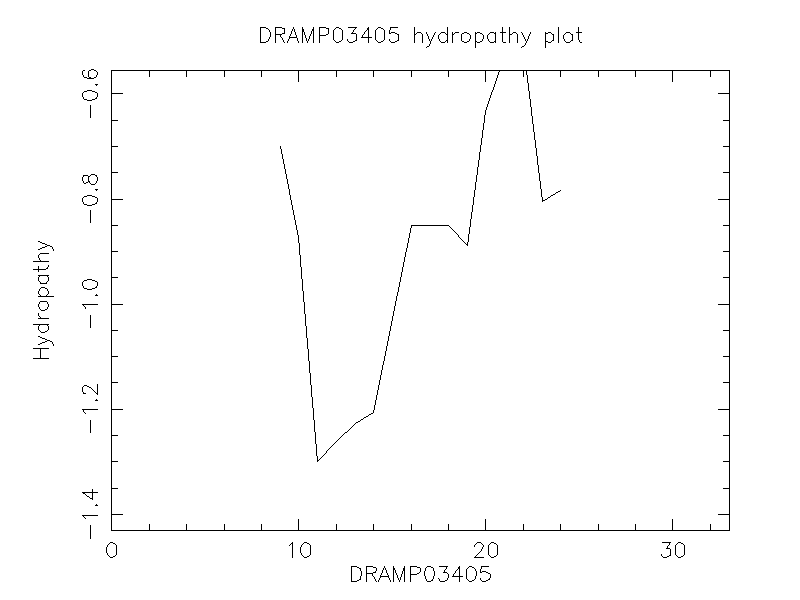
Hydrophobicity
- -0.815
Aliphatic Index
- 91.52
Half Life
-
- Mammalian:30 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 6
DRAMP03405
Comments Information
Function
- Acts as a potent antimicrobial peptide.
Tissue specificity
- Expressed in testis, spleen, stomach, and intestine. Very low expression found in heart, lung and skeletal muscle. No expression in brain, kidney or liver.
Literature Information
- ·Literature 1
-
Title
- Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse.
-
Pubmed ID
- 9148921
-
Reference
- J Biol Chem. 1997 May 16;272(20):13088-13093.
-
Author
- Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R.
- ·Literature 2
-
Title
- A novel murine cathelin-like protein expressed in bone marrow.
-
Pubmed ID
- 8706928
-
Reference
- FEBS Lett. 1996 Aug 5;391(1-2):5-8.
-
Author
- Popsueva AE, Zinovjeva MV, Visser JW, Zijlmans JM, Fibbe WE, Belyavsky AV.