General Information
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-, Antifungal
-
Target Organism
-
- Gram-negative bacterium: Escherichia coli;
- Gram-positive bacteria: Staphylococcus aureus, S. epidermidis, group B Streptococcus.
- Fungi: Candida albicans, Aspergillus fumigatus, Aspergillus niger.
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
Structure
- Beta strand
-
Structure Description
- Not found
-
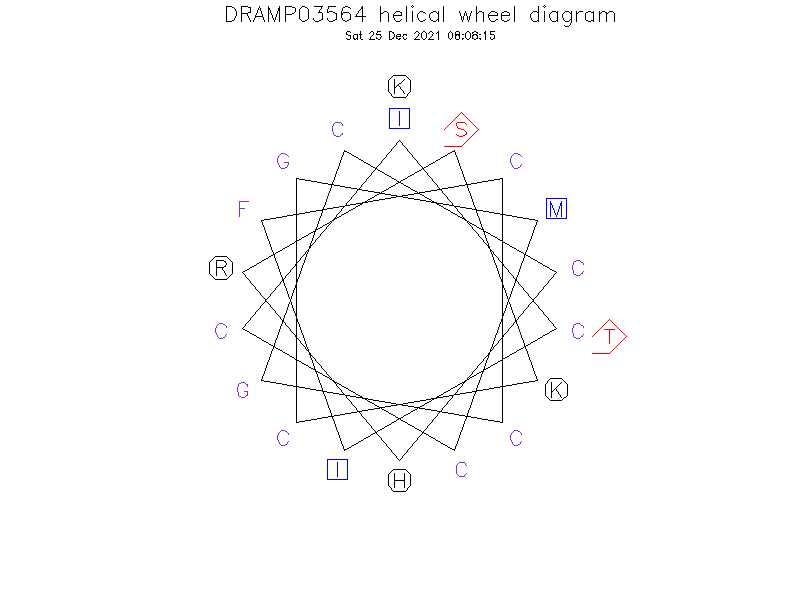
Helical Wheel Diagram
-
PDB ID
- 1M4E resolved by NMR.
-
Predicted Structure
- There is no predicted structure for DRAMP03564.
Physicochemical Information
-
Formula
- C85H143N27O23S9
Absent Amino Acids
- ADELNPQVWY
Common Amino Acids
- C
Mass
- 2199.78
PI
- 8.53
Basic Residues
- 4
Acidic Residues
- 0
Hydrophobic Residues
- 3
Net Charge
- +4
-
Boman Index
- -9.36
Hydrophobicity
- 0.795
Aliphatic Index
- 39
Half Life
-
- Mammalian:20 hour
- Yeast:30 min
- E.coli:>10 hour
Extinction Coefficient Cystines
- 500
Absorbance 280nm
- 26.32
Polar Residues
- 12
DRAMP03564
Comments Information
Function
- Seems to act as a signaling molecule involved in the maintenance of iron homeostasis. Seems to be required in conjunction with HFE to regulate both intestinal iron absorption and iron storage in macrophages. Has strong antimicrobial activity against E.coli ML35P N.cinerea and weaker against S.epidermidis, S.aureus and group b streptococcus bacteria. Active against the fungus C.albicans. No activity against P.aeruginosa.
Tissue specificity
- Highest expression in liver and to a lesser extent in heart and brain. Low levels in lung, tonsils, salivary gland, trachea, prostate gland, adrenal gland and thyroid gland. Secreted into the urine.
PTM
- Contains four disulfide bonds 2-18; 5-17; 6-14; 8-9.
Literature Information
- ·Literature 1
-
Title
- Hepcidin, a urinary antimicrobial peptide synthesized in the liver.
-
Pubmed ID
- 11113131
-
Reference
- J Biol Chem. 2001 Mar 16;276(11):7806-7810.
-
Author
- Park CH, Valore EV, Waring AJ, Ganz T.
- ·Literature 2
-
Title
- The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis.
-
Pubmed ID
- 12138110
-
Reference
- J Biol Chem. 2002 Oct 4;277(40):37597-37603.
-
Author
- Hunter HN, Fulton DB, Ganz T, Vogel HJ.
- ·Literature 3
-
Title
- LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. ref2) Hepcidin, a rinary antimicrobial peptide synthesized in the liver.
-
Pubmed ID
- 11034317
-
Reference
- FEBS Lett. 2000 Sep 1;480(2-3):147-150.
-
Author
- Krause A et al Adermann K.