General Information
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-, Antifungal
-
Target Organism
-
- Gram-negative bacterium: Escherichia coli DC2 (MIC=9 µM);
- Gram-positive bacteria: Staphylococcus aureus mprF (presence of carbonate) (MIC=3 µM), S. aureu mprF (absence of carbonate) (MIC=24 µM).
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Free
-
Nonterminal Modifications and Unusual Amino Acids
- Free
-
Stereochemistry
- L
-
Structure
- Alpha helix (1 helices; 19 residues)
-
Structure Description
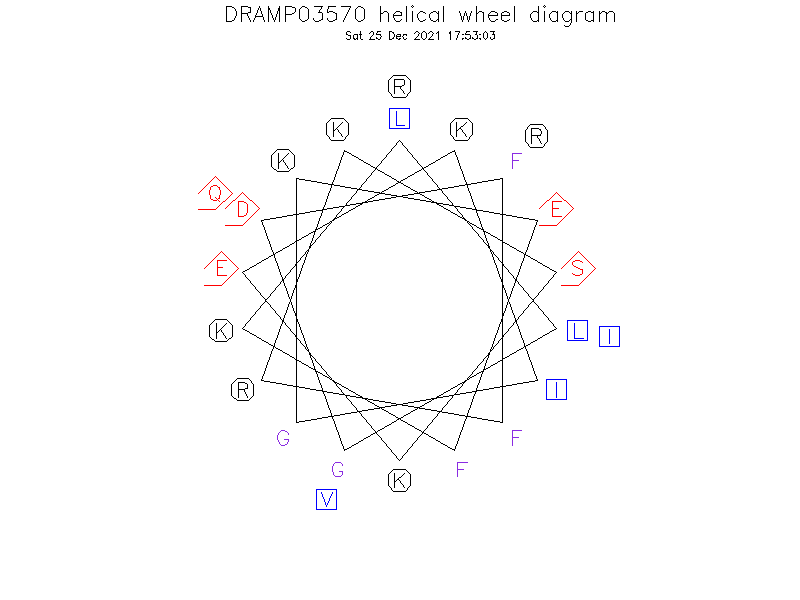
- The Ser9 site splits the hydrophobic surface of the amphipathic helix into two domains, which explains its poor antimicrobial activity (active against only susceptible bacterial strains).
-
Helical Wheel Diagram
-
PDB ID
- 2LMF resolved by NMR.
-
Predicted Structure
- There is no predicted structure for DRAMP03570.
Physicochemical Information
-
Formula
- C130H216N38O32
Absent Amino Acids
- ACHMNPTWY
Common Amino Acids
- K
Mass
- 2823.38
PI
- 10.55
Basic Residues
- 8
Acidic Residues
- 3
Hydrophobic Residues
- 8
Net Charge
- +5
-
Boman Index
- -69.25
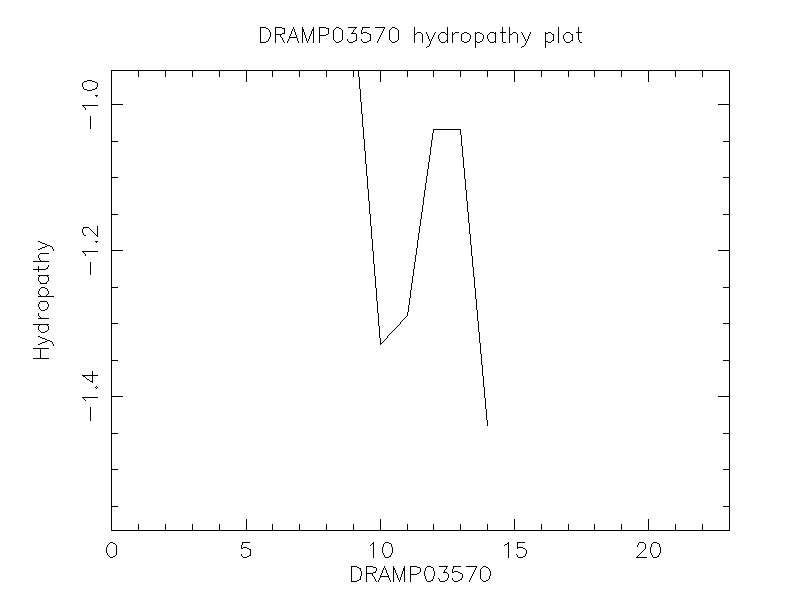
Hydrophobicity
- -0.844
Aliphatic Index
- 80.43
Half Life
-
- Mammalian:5.5 hour
- Yeast:3 min
- E.coli:2 min
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 3
DRAMP03570
Comments Information
Its structure, dynamics, and antimicrobial and immune modulating activities have been characterized (Biochemistry 2012).
Literature Information
- ·Literature 1
-
Title
- Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin.
-
Pubmed ID
- 17012259
-
Reference
- FASEB J 2006; 20, 2068-2080.
-
Author
- Yamasaki K et al. Gallo RL.
- ·Literature 2
-
Title
- Structure, dynamics, and antimicrobial and immune modulatory activities of human LL-23 and its single-residue variants mutated on the basis of homologous primate cathelicidins.
-
Pubmed ID
- 22185690
-
Reference
- Biochemistry. 2012 Jan 17;51(2):653-664.
-
Author
- Wang G, Elliott M, Cogen AL, Ezell EL, Gallo RL, Hancock RE.