General Information
-
DRAMP ID
- DRAMP03646
-
Peptide Name
- Cathelicidin-3 (CATH-3; Fowlicidin-3; Birds, animals)
-
Source
- Gallus gallus (Chicken)
-
Family
- Not found
-
Gene
- CATHL3
-
Sequence
- RVKRFWPLVPVAINTVAAGINLYKAIRRK
-
Sequence Length
- 29
-
UniProt Entry
- Q2IAL6
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-
-
Target Organism
-
- Gram-negative bacteria: Escherichia coli 25922 (MIC=2 µM), Salmonella typhimurium 14028 (MIC=2 µM), S. typhimurium DT104 700408 (MIC=2 µM), Salmonella enteritidis 13076 (MIC=2 µM), Klebsiella pneumoniae 13883 (MIC=1 µM);
- Gram-positive bacteria: Listeria monocytogenes 19115 (MIC=2 µM), Staphylococcus aureus 25923 (MIC=1 µM), S. aureus (MRSA) 43300 (MIC=1 µM), S. aureus (MRSA) BAA-39 (MIC=1 µM).
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Lipopolysaccharide (LPS)-binding
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Free
-
Nonterminal Modifications and Unusual Amino Acids
- Free
-
Stereochemistry
- L
-
Structure
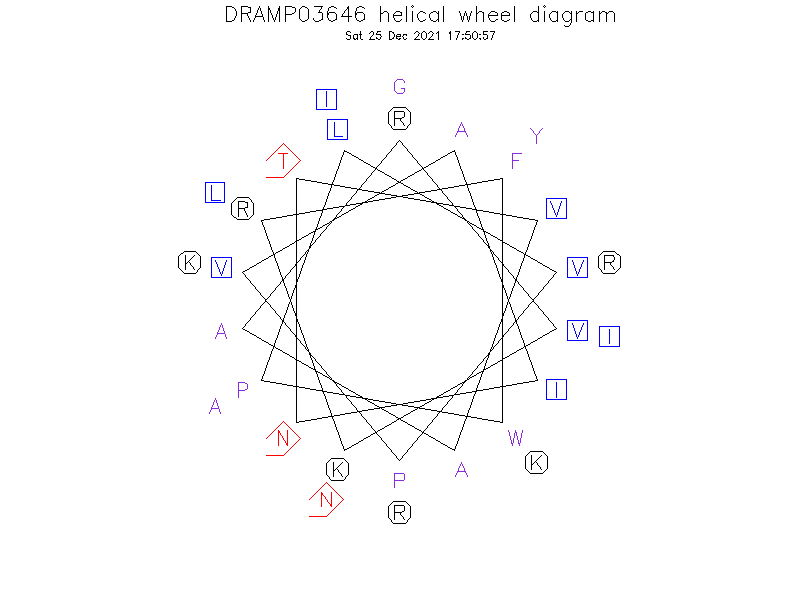
- Alpha helix (1 helices; 9 residues)
-
Structure Description
- NMR spectroscopy reveales that fowlicidin-3 comprises 27 amino-acid residues and adopts a predominantly alpha-helical structure extending from residue 9 to 25 with a slight kink induced by a glycine at position 17..
-
Helical Wheel Diagram
-
PDB ID
- 2HFR resolved by NMR.
-
Predicted Structure
- There is no predicted structure for DRAMP03646.
Physicochemical Information
-
Formula
- C157H261N47O34
Absent Amino Acids
- CDEHMQS
Common Amino Acids
- ARV
Mass
- 3351.09
PI
- 12.02
Basic Residues
- 7
Acidic Residues
- 0
Hydrophobic Residues
- 15
Net Charge
- +7
-
Boman Index
- -38.07
Hydrophobicity
- 0.162
Aliphatic Index
- 121.03
Half Life
-
- Mammalian:1 hour
- Yeast:2 min
- E.coli:2 min
Extinction Coefficient Cystines
- 6990
Absorbance 280nm
- 249.64
Polar Residues
- 5
DRAMP03646
Comments Information
Function
- May have antimicrobial activity and play a role in the innate immune response.
Tissue specificity
- Detected in bone marrow, liver and lung.
Literature Information
- ·Literature 1
-
Title
- Chicken cathelicidin-B1, an antimicrobial guardian at the mucosal M cell gateway.
-
Pubmed ID
- 17827276
-
Reference
- Proc Natl Acad Sci U S A. 2007 Sep 18;104(38):15063-15068.
-
Author
- Goitsuka R, Chen CL, Benyon L, Asano Y, Kitamura D, Cooper MD.
- ·Literature 2
-
Title
- Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity.
-
Pubmed ID
- 16326712
-
Reference
- J Biol Chem. 2006 Feb 3;281(5):2858-2867.
-
Author
- Xiao Y, Cai Y, Bommineni YR, Fernando SC, Prakash O, Gilliland SE, Zhang G.
- ·Literature 3
-
Title
- Fowlicidin-3 is an alpha-helical cationic host defense peptide with potent antibacterial and lipopolysaccharide-neutralizing activities.
-
Pubmed ID
- 17229147
-
Reference
- FEBS J. 2007 Jan;274(2):418-428.
-
Author
- Bommineni YR, Dai H, Gong YX, Soulages JL, Fernando SC, Desilva U, Prakash O, Zhang G.