General Information
-
DRAMP ID
- DRAMP03724
-
Peptide Name
- Pandinin-2 (Pin2; Arthropods, animals)
-
Source
- Pandinus imperator (Emperor scorpion)
-
Family
- Belongs to the scorpion NDBP 4 family
-
Gene
- Not found
-
Sequence
- FWGALAKGALKLIPSLFSSFSKKD
-
Sequence Length
- 24
-
UniProt Entry
- P83240
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-, Hemolytic
-
Target Organism
-
- [Ref.11563967]Gram-negative bacteria: Pseudomonas aeruginosa (MIC=38.2 µM), Escherichia coli (MIC=19.1 µM);#Gram-positive bacteria: Enterococcus faecalis (MIC=2.4 µM), Bacillus subtilis (MIC=4.8 µM), Staphylococcus epidermidis (MIC=4.8 µM), Staphylococcus aureus (MIC=2.4 µM);
- Yeast: Candida albicans (MIC=19.1 µM).
-
Hemolytic Activity
-
- [Ref.11563967]Strong hemolytic activity (11.1-44.5 microM) against sheep erythrocytes
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Cell membrane
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Free
-
Nonterminal Modifications and Unusual Amino Acids
- Free
-
Stereochemistry
- L
-
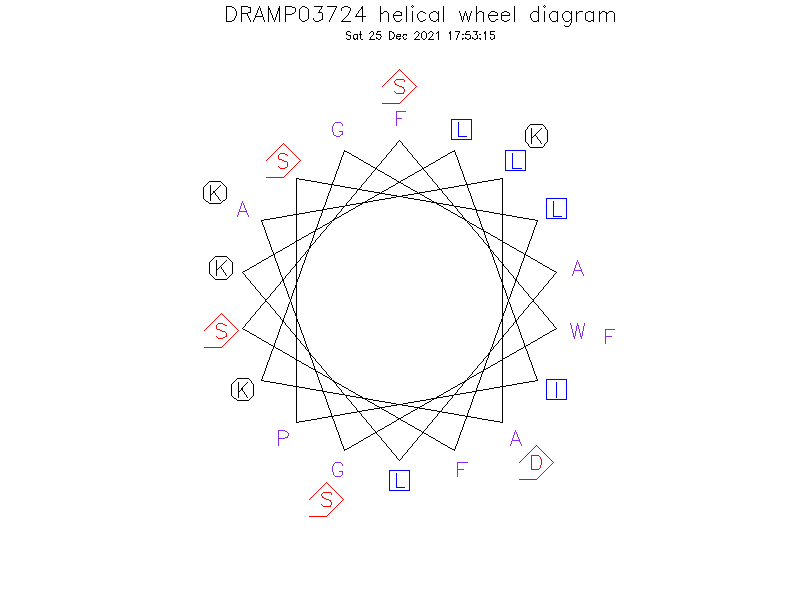
Structure
- Alpha helix
-
Structure Description
- Not found
-
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP03724.
Physicochemical Information
-
Formula
- C126H195N29O31
Absent Amino Acids
- CEHMNQRTVY
Common Amino Acids
- KLS
Mass
- 2612.11
PI
- 10
Basic Residues
- 4
Acidic Residues
- 1
Hydrophobic Residues
- 12
Net Charge
- +3
-
Boman Index
- -1.34
Hydrophobicity
- 0.329
Aliphatic Index
- 93.75
Half Life
-
- Mammalian:1.1 hour
- Yeast:3 min
- E.coli:2 min
Extinction Coefficient Cystines
- 5500
Absorbance 280nm
- 239.13
Polar Residues
- 6
DRAMP03724
Comments Information
Function
- Disrupts cell membranes through formation of pores. Has strong antimicrobial activity against Gram-positive bacteria and less active against Gram-negative bacteria. Possesses antifungal activity against C. albicans and hemolytic activity against sheep and pig erythrocytes.
Tissue specificity
- Expressed by the venom gland.
Literature Information
- ·Literature 1
-
Title
- Characterization of unique amphipathic antimicrobial peptides from venom of the scorpion Pandinus imperator.
-
Pubmed ID
- 11563967
-
Reference
- Biochem J. 2001 Oct 1;359(Pt 1):35-45.
-
Author
- Corzo G, Escoubas P, Villegas E, Barnham KJ, He W, Norton RS, Nakajima T.
- ·Literature 2
-
Title
- Pore formation of phospholipid membranes by the action of two hemolytic arachnid peptides of different size.
-
Pubmed ID
- 15328050
-
Reference
- Biochim Biophys Acta. 2004 Aug 30;1664(2):182-188.
-
Author
- Belokoneva OS, Satake H, Mal'tseva EL, Pal'mina NP, Villegas E, Nakajima T, Corzo G.
- ·Literature 3
-
Title
- Orientation and pore-forming mechanism of a scorpion pore-forming peptide bound to magnetically oriented lipid bilayers.
-
Pubmed ID
- 15298871
-
Reference
- Biophys J. 2004 Oct;87(4):2497-507.
-
Author
- Nomura K, Corzo G, Nakajima T, Iwashita T.