General Information
-
DRAMP ID
- DRAMP03828
-
Peptide Name
- Novispirin T-7 (mutation of Ovispirin-1)
-
Source
- Synthetic construct
-
Family
- Not found
-
Gene
- Not found
-
Sequence
- KNLRRITRKIIHIIKKYG
-
Sequence Length
- 18
-
UniProt Entry
- No entry found
-
Protein Existence
- Synthetic
Activity Information
-
Biological Activity
- Antibacterial, Cytotoxic, Anti-Gram+, Anti-Gram-, Antimicrobial
-
Target Organism
-
- [Ref.11932493]Gram-negative bacteria: Pseudomonas aeruginosa 9 strains (MIC=0.26-27.8 µg/ml), Stenotrophomonas maltophilia 5 strains (MIC=0.32-1.75 µg/ml);
- Gram-positive bacteria: Listeria monocytogenes EGD (MIC=2.4±1.5 [n=3] µg/ml), Staphylococcus aureus 930918 (MIC=3.3±1.5 [n=3] µg/ml).
-
Hemolytic Activity
-
- [Ref.11932493]3% hemolytic at 40 µg/mL, 10% hemolytic activity at 80 µg/mL against human red blood cells
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Free
-
Nonterminal Modifications and Unusual Amino Acids
- Free
-
Stereochemistry
- L
-
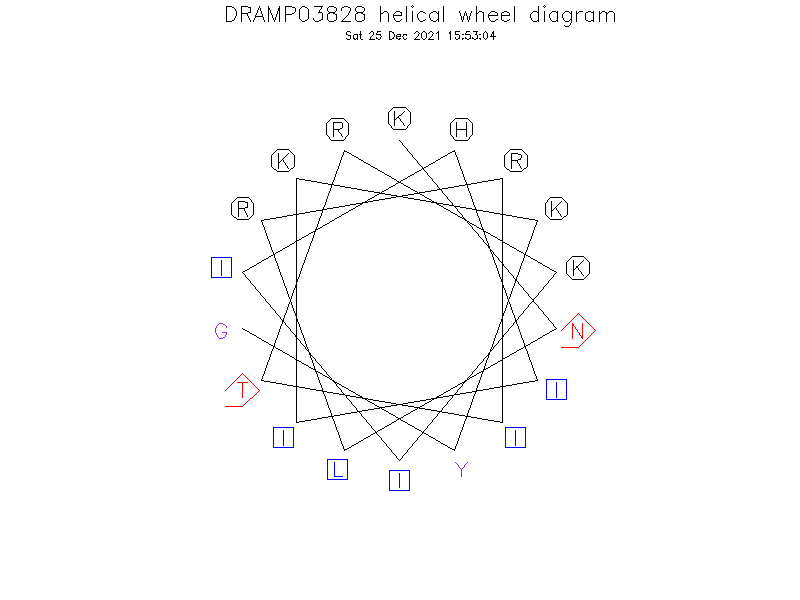
Structure
- Alpha helix (1 helices; 11 residues)
-
Structure Description
- Novispirin T-7 is a well-defined α-helix over residues 7-17 with residues 3-6 forming a type IV turn.
-
Helical Wheel Diagram
-
PDB ID
- 1HU7 resolved by NMR.
-
Predicted Structure
- There is no predicted structure for DRAMP03828.
Physicochemical Information
-
Formula
- C103H184N34O22
Absent Amino Acids
- ACDEFMPQSVW
Common Amino Acids
- I
Mass
- 2250.81
PI
- 11.75
Basic Residues
- 8
Acidic Residues
- 0
Hydrophobic Residues
- 6
Net Charge
- +8
-
Boman Index
- -50.51
Hydrophobicity
- -0.661
Aliphatic Index
- 130
Half Life
-
- Mammalian:1.3 hour
- Yeast:3 min
- E.coli:2 min
Extinction Coefficient Cystines
- 1490
Absorbance 280nm
- 87.65
Polar Residues
- 4
DRAMP03828
Comments Information
Function
- Possess potently antimicrobial activity. Also has hemolytic activity against Human red blood cells, and has cytotoxicity activity against ME-180 cervical cells (81.5% at 200 µg/ml, 28.4% at 50 µg/ml, 15.6% at 25 µg/ml) and A-549 pulmonary cells (81.5% at 200 µg/ml, 22.9% at 50 µg/ml, 15.4% at 25 µg/ml).
Literature Information
- ·Literature 1
-
Title
- Impact of single-residue mutations on the structure and Function: of ovispirin/novispirin antimicrobial peptides.
-
Pubmed ID
- 11932493
-
Reference
- Protein Eng. 2002 Mar;15(3):225-232.
-
Author
- Sawai MV, Waring AJ, Kearney WR, McCray PB Jr, Forsyth WR, Lehrer RI, Tack BF.