General Information
-
DRAMP ID
- DRAMP03962
-
Peptide Name
- Cecropin A(1-8)-Magainin 2(1-12)hybrid peptide (CAMA)
-
Source
- Synthetic construct
-
Family
- Not found
-
Gene
- Not found
-
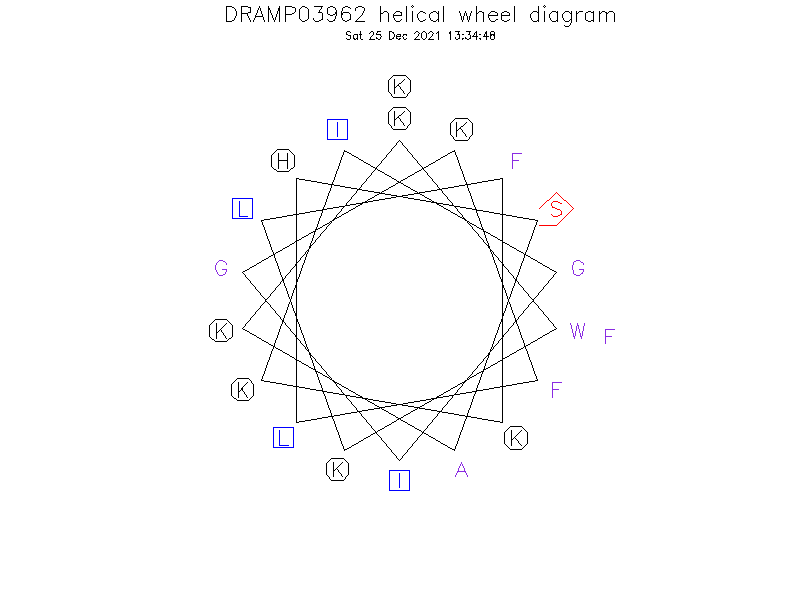
Sequence
- KWKLFKKIGIGKFLHSAKKF
-
Sequence Length
- 20
-
UniProt Entry
- P01507
-
Protein Existence
- Synthetic
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial
-
Target Organism
- No MICs found in DRAMP database
-
Hemolytic Activity
-
- No hemolysis information or data found in the reference(s) presented in this entry
-
Cytotoxicity
-
- Not included yet
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Not included yet
-
N-terminal Modification
- Not included yet
-
C-terminal Modification
- Not included yet
-
Nonterminal Modifications and Unusual Amino Acids
- Not included yet
-
Stereochemistry
- Not included yet
-
Structure
- Alpha helix
-
Structure Description
- Not found
-
Helical Wheel Diagram
-
Predicted Structure
- There is no predicted structure for DRAMP03962.
Physicochemical Information
-
Formula
- C120H190N30O22
Absent Amino Acids
- CDEMNPQRTVY
Common Amino Acids
- K
Mass
- 2405.02
PI
- 10.78
Basic Residues
- 8
Acidic Residues
- 0
Hydrophobic Residues
- 9
Net Charge
- +8
-
Boman Index
- -12.27
Hydrophobicity
- -0.31
Aliphatic Index
- 83
Half Life
-
- Mammalian:1.3 hour
- Yeast:3 min
- E.coli:2 min
Extinction Coefficient Cystines
- 5500
Absorbance 280nm
- 289.47
Polar Residues
- 3
DRAMP03962
Comments Information
MOA
- The partial insertion of the Trp2 of CA-MA into the membrane, as well as the electrostatic interactions between the positively charged Lys residues at the N-terminus of the CA-MA and the anionic phospholipid headgroups, leads to the primary binding to the cell membrane. Then, the flexibility or bending potential induced by the Gly-Ile-Gly hinge sequence or the Pro residue in the central part of the peptides may allow the alpha-helix in the C-terminus to span the lipid bilayer.
Literature Information
- ·Literature 1
-
Title
- Role of the hinge region and the tryptophan residue in the synthetic antimicrobial peptides, cecropin A(1-8)-magainin 2(1-12) and its analogues, on their antibiotic activities and structures.
-
Pubmed ID
- 11009597
-
Reference
- Biochemistry. 2000 Oct 3;39(39):11855-11864.
-
Author
- Oh D, Shin SY, Lee S, Kang JH, Kim SD, Ryu PD, Hahm KS, Kim Y.