General Information
-
DRAMP ID
- DRAMP20862
-
Peptide Name
- rtCATH2(5-40)
-
Source
- Synthetic construct
-
Family
- Derived from Cathelicidins
-
Gene
- Not found
-
Sequence
- RRGKDSGGPKMGRKNSKGGWRGRPGSGRPGFGSGI
-
Sequence Length
- 39
-
UniProt Entry
- No entry found
-
Protein Existence
- Synthetic
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-
-
Target Organism
-
- [Ref.27818338]Gram-negative bacteria:Escherichia coli (ATCC 25922)(MIC=16μM);A. salmonicida (ATCC 33658)(MIC=16μM);Y. ruckeri (NCIMB 1315)(MIC=16μM);
- Gram-positive bacteria:Staphylococcus aureus (ATCC 25923)(MIC=64μM)
-
Hemolytic Activity
-
- [Ref.27818338] No hemolytic activity to human
-
Cytotoxicity
- No cytotoxicity information found in the reference(s) presented
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Free
-
Nonterminal Modifications and Unusual Amino Acids
- None
-
Stereochemistry
- L
-
Structure
- Unconventional feature
-
Structure Description
- Not found
-
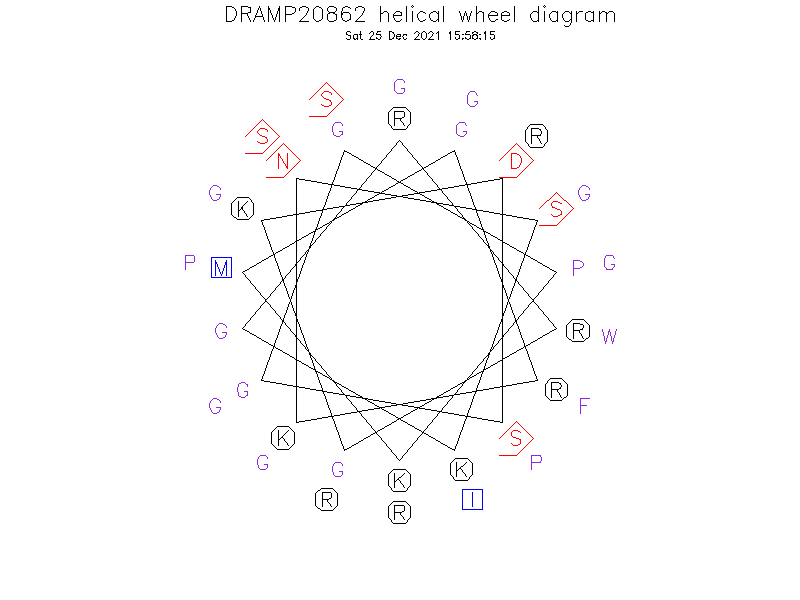
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP20862.
Physicochemical Information
-
Formula
- C150H249N59O43S
Absent Amino Acids
- ACEHLQTVY
Common Amino Acids
- G
Mass
- 3599.06
PI
- 12.31
Basic Residues
- 10
Acidic Residues
- 1
Hydrophobic Residues
- 3
Net Charge
- +9
-
Boman Index
- -11682
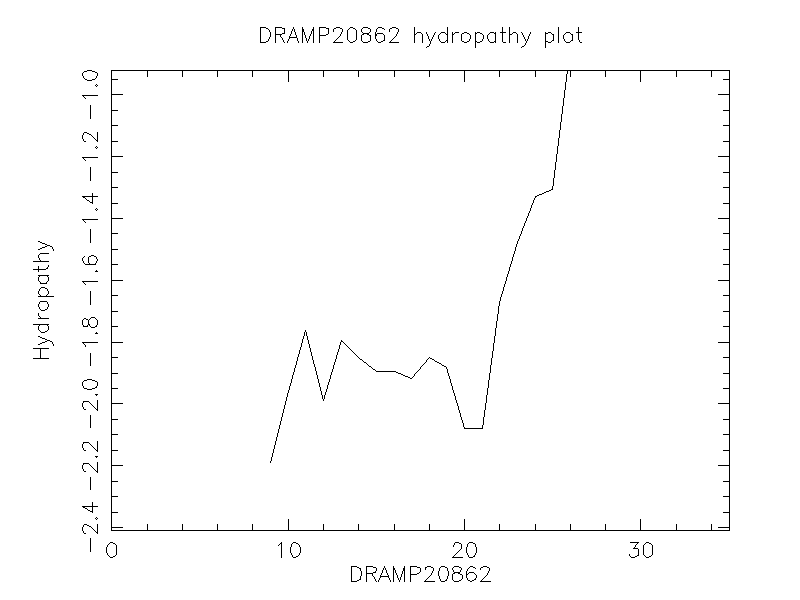
Hydrophobicity
- -1.546
Aliphatic Index
- 11.14
Half Life
-
- Mammalian:1 hour
- Yeast:2 min
- E.coli:2 min
Extinction Coefficient Cystines
- 5500
Absorbance 280nm
- 161.76
Polar Residues
- 17
DRAMP20862
Comments Information
Function
- Antibacterial activity against the Gram-positive and Gram-negative bacteria.The peptides inhibit the growth of V. anguillarum or A. hydrophila (MIC >32
Literature Information
- ·Literature 1
-
Title
- Antimicrobial and host cell-directed activities of Gly/Ser-rich peptides from salmonid cathelicidins.
-
Pubmed ID
- 27818338
-
Reference
- Fish Shellfish Immunol. 2016 Dec;59:456-468.
-
Author
- D'Este F, Benincasa M, Cannone G, Furlan M, Scarsini M, Volpatti D, Gennaro R, Tossi A, Skerlavaj B, Scocchi M.