General Information
-
DRAMP ID
- DRAMP21211
-
Peptide Name
- AmyI-1-18 (N3L, G12R) (Derived from AmyI-1-18)
-
Source
- Synthetic construct
-
Family
- Not found
-
Gene
- Not found
-
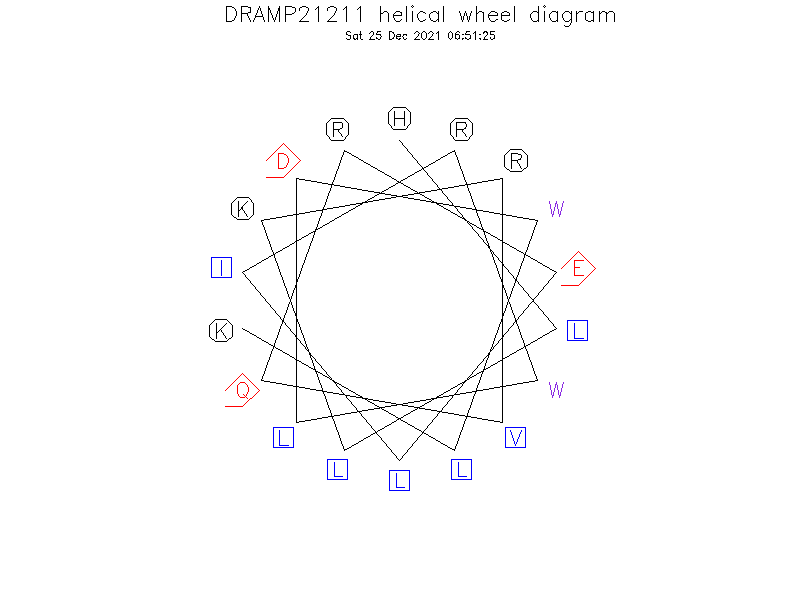
Sequence
- HLLKRVQRELIRWLDWLK
-
Sequence Length
- 18
-
UniProt Entry
- No entry found
-
Protein Existence
- Synthetic form
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram-
-
Target Organism
-
- [Ref.27478151] Gram-negative bacteria : Porphyromonas gingivalis(IC50=2.9±0.2 μM)
-
Hemolytic Activity
-
- [Ref.27478151] 14% hemolysis at 50 μM against human red blood cells
-
Cytotoxicity
- No cytotoxicity information found in the reference(s) presented
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Free
-
Nonterminal Modifications and Unusual Amino Acids
- None
-
Stereochemistry
- L
-
Structure
- Alpha helix
-
Structure Description
- Not found
-
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP21211.
Physicochemical Information
-
Formula
- C113H184N34O24
Absent Amino Acids
- ACFGMNPSTY
Common Amino Acids
- L
Mass
- 2402.92
PI
- 10.9
Basic Residues
- 6
Acidic Residues
- 2
Hydrophobic Residues
- 9
Net Charge
- +4
-
Boman Index
- -4337
Hydrophobicity
- -0.506
Aliphatic Index
- 146.11
Half Life
-
- Mammalian:3.5 hour
- Yeast:10 min
- E.coli:>10 hour
Extinction Coefficient Cystines
- 11000
Absorbance 280nm
- 647.06
Polar Residues
- 0
DRAMP21211
Comments Information
Function
- Antibacterial activity against Gram-negative bacteria
Literature Information
- ·Literature 1
-
Title
- Antimicrobial activity against Porphyromonas gingivalis and mechanism of action of the cationic octadecapeptide AmyI-1-18 and its amino acid-substituted analogs.
-
Pubmed ID
- 27478151
-
Reference
- J Biosci Bioeng. 2016 Dec;122(6):652-659. doi: 10.1016/j.jbiosc.2016.05.008.
-
Author
- Taniguchi M, Ochiai A, Takahashi K, Nakamichi SI, Nomoto T, Saitoh E, Kato T, Tanaka T