General Information
-
DRAMP ID
- DRAMP21337
-
Peptide Name
- BMAP-27 (Bovine, mammals, animals)
-
Source
- Bos taurus (Bovine)
-
Family
- Belongs to the cathelicidin family
-
Gene
- CATHL6
-
Sequence
- GRFKRFRKKFKKLFKKLSPVIPLLHL
-
Sequence Length
- 26
-
UniProt Entry
- P54228
-
Protein Existence
- Protein level
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-
-
Target Organism
-
- [Ref.31226350] Gram-positive bacteria:Staphylococcus aureus (MIC=1-2 μM);
- Gram-negative bacteria: Escherichia coli (MIC=2-4 μM)
-
Hemolytic Activity
-
- [Ref.31226350] 50% hemolysis at 75 μM for human red blood cells
-
Cytotoxicity
-
- [Ref.31226350] The CC50 values which represent peptide concentration (μM) inducing 50% cytotoxicity to human cancer cell lines of BMAP-27 on MDA361 and A549 are 4.2 μM and 5.3 μM. DETAILED DATA: ①The cell viability of MDA-361 induced by BMAP-27 is 100%, 100%, 98.9%, 97.7%, 87.6%, 59.8%, 34.7%, 12.4%, 10.3%, 8.3% and 9.4% at peptide concentrations of 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 13, 25, 50 and 100 μM. ②The cell viability of A549 induced by BMAP-27 is 100%, 99.1%, 97.7%, 94.7%, 74.5%, 41.4%, 24.4%, 11.3%, 9.7%, 10.3% and 7.4% at peptide concentrations of 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 13, 25, 50 and 100 μM.
-
Binding Target
- Not found
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Amidation
-
Nonterminal Modifications and Unusual Amino Acids
- None
-
Stereochemistry
- L
-
Structure
- 73% α-helix in 50% TFE/H2O
-
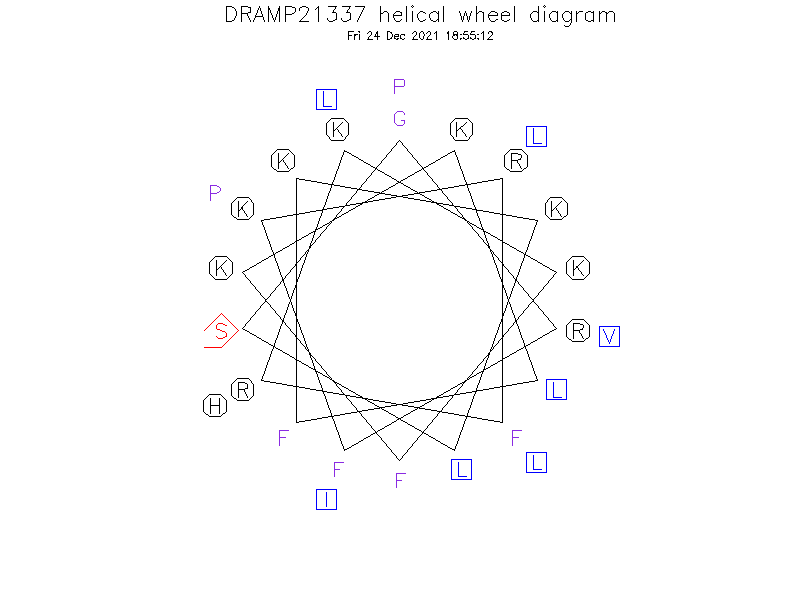
Structure Description
- An α-helix occurred from Arg2 to Leu17 in the N-terminal region and a short α-helix is observed from Leu23 to Leu26 in the Cterminal region; the two regions are connected with a flexible region containing two proline residues.
-
Helical Wheel Diagram
-
PDB ID
- 2KET resolved by NMR
-
Predicted Structure
- There is no predicted structure for DRAMP21337.
Physicochemical Information
-
Formula
- C158H262N44O28
Absent Amino Acids
- ACDEMNQTWY
Common Amino Acids
- K
Mass
- 3226.1
PI
- 12.32
Basic Residues
- 11
Acidic Residues
- 0
Hydrophobic Residues
- 11
Net Charge
- +11
-
Boman Index
- -4525
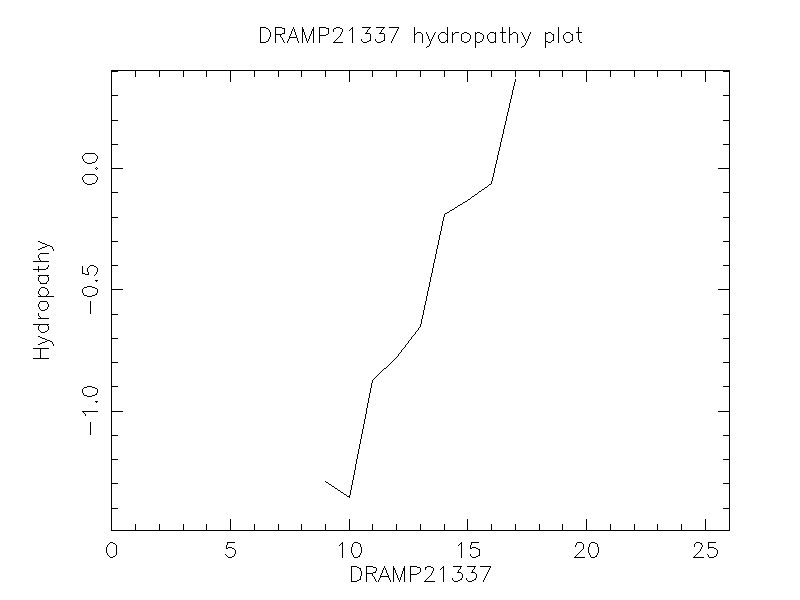
Hydrophobicity
- -0.365
Aliphatic Index
- 101.15
Half Life
-
- Mammalian:30 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 2
DRAMP21337
Comments Information
Function
- Antibacterial activity against Gram-positive bacteria and Gram-negative bacteria.
Literature Information
- ·Literature 1
-
Title
- Structural analysis and mode of action of BMAP-27, a cathelicidin-derived antimicrobial peptide
-
Pubmed ID
- 31226350
-
Reference
- Peptides. 2019 Jun 18;118:170106. doi: 10.1016/j.peptides.2019.170106.
-
Author
- Yang S, Lee CW, Kim HJ, Jung HH, Kim JI, Shin SY, Shin SH