General Information
-
DRAMP ID
- DRAMP29049
-
Peptide Name
- Ano-1β (variant of Anoplin)
-
Source
- Synthetic construct (Ano-1β is a variant of Anoplin, which is a linear cationic α-helical AMPs isolated from the venom sac of anoplius samariensis(solitary spider wasps))
-
Family
- Not found
-
Gene
- N/A
-
Sequence
- ALLKRIKTLL
-
Sequence Length
- 10
-
UniProt Entry
- No entry found
-
Protein Existence
- Synthetic form
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-
-
Target Organism
-
- [Ref.32654770] Gram-negative multidrug-resistant bacteria: P. aeruginosa 119 (MIC = 8 μM or 8.93 μg/mL), P. aeruginosa 124 (MIC = 8 μM or 8.93 μg/mL), P. aeruginosa 86 (MIC = 4 μM or 4.47 μg/mL);
- Gram-negative bacteria: P. aeruginosa ATCC 27853 (MIC = 8 μM or 8.93 μg/mL), P. aeruginosa ATCC 9027 (MIC = 8 μM or 8.93 μg/mL), E. coli ATCC 25922 (MIC = 32 μM or 35.72 μg/mL), K. pneumonia ATCC 700603 (MIC = 32 μM or 35.72 μg/mL), A. baumannii ATCC 19606 (MIC = 64 μM or 71.44 μg/mL);
- Gram-positive bacteria: S. aureus ATCC 25923 (MIC = 128 μM or 142.88 μg/mL), S. epidermidis ATCC 12228 (MIC = 8 μM or 8.93 μg/mL)
-
Hemolytic Activity
-
- [Ref.32654770]Ano-1β induced inappreciable hemolytic activity with less than 5 % hemolysis at all the tested concentrations and HC > 256 μM. Note: Minimum hemolysis concentration (HC, μM) was defined as the minimal peptide concentration induced 10 % hemolysis.
-
Cytotoxicity
-
- [Ref.32654770]After 1 h of incubation, Ano-1β, similar to their parent peptide anoplin, maintained more than 80 % cell viability of the two cells at all tested concentrations. After incubating for 24 h, Ano-1β still maintained more than 80 % cell viability of HEK 293 T cells at all tested concentrations but displayed cytotoxicity against Hela cells at the highest concentration (256 μM). The cell viability of Hela after 24 h of incubation is around 80%.
-
Binding Target
- Membrane
Structure Information
-
Linear/Cyclic
- Linear
-
N-terminal Modification
- Free
-
C-terminal Modification
- Amidation
-
Nonterminal Modifications and Unusual Amino Acids
- [Ref.32654770] The residue A at position 1 indicates the β-Ala
-
Stereochemistry
- L
-
Structure
- 75.3% α-helical content in 30 mM SDS
-
Structure Description
- As shown in Fig. 1, the new peptides displayed mostly unordered conformations in the aqueous environment as demonstrated by a negative peak at approximately 195 nm. However, in 30 mM SDS, the new peptides folded typical α-helical conformations, as evidenced by a characteristic positive peak at approximately 195 nm and double negative peaks at approximately 208 nm and 222 nm.
-
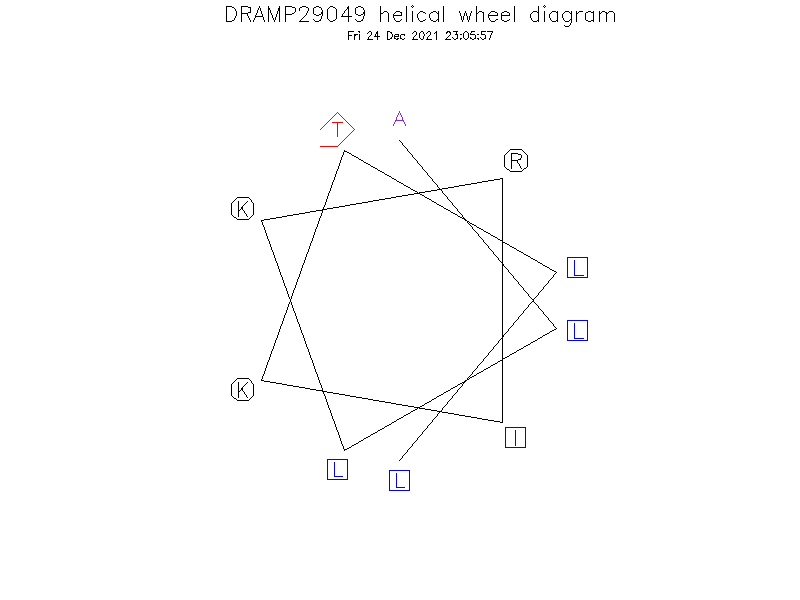
Helical Wheel Diagram
-
PDB ID
- None
-
Predicted Structure
- There is no predicted structure for DRAMP29049.
Physicochemical Information
-
Formula
- C55H105N15O12
Absent Amino Acids
- CDEFGHMNPQSVWY
Common Amino Acids
- L
Mass
- 1168.53
PI
- 11.17
Basic Residues
- 3
Acidic Residues
- 0
Hydrophobic Residues
- 6
Net Charge
- +3
-
Boman Index
- -218
Hydrophobicity
- 0.85
Aliphatic Index
- 205
Half Life
-
- Mammalian:4.4 hour
- Yeast:>20 hour
- E.coli:>10 hour
Extinction Coefficient Cystines
- 0
Absorbance 280nm
- 0
Polar Residues
- 1
DRAMP29049
Comments Information
Comment
- the incorporation of β-Ala had significant influence on the antimicrobial activity of peptides mainly due to the changes of hydrophobicity and net positive charge. Ano-1β exhibited good antimicrobial potency, which was stable under physiological conditions and displayed preferable in vivo antimicrobial activity with less acute toxicity. In particular, Ano-1β showed low tendency to develop bacterial resistance in contrast to conventional antibiotics rifampicin and gentamicin, and they exhibited better anti-biofilm activity and synergistic or additive effects in combination with conventional antibiotics. Ano-1β might stands as a promising antimicrobial candidate to overcome increasing bacterial resistance, and the incorporation of β-Ala was a reasonable strategy for the development of promising antimicrobial agents.
Literature Information
- ·Literature 1
-
Title
- Synthesis and anti-pseudomonal activity of new ß-Ala modified analogues of the antimicrobial peptide anoplin
-
Pubmed ID
- 32654770
-
Reference
- Int J Med Microbiol. 2020 Jul;310(5):151433. doi: 10.1016/j.ijmm.2020.151433. Epub 2020 May 27.
-
Author
- Zhong C, Zhu Y, Zhu N, Liu T, Gou S, Zhang F, Yao J, Xie J, Ni J