-
-
-
-
-
Original Sequence
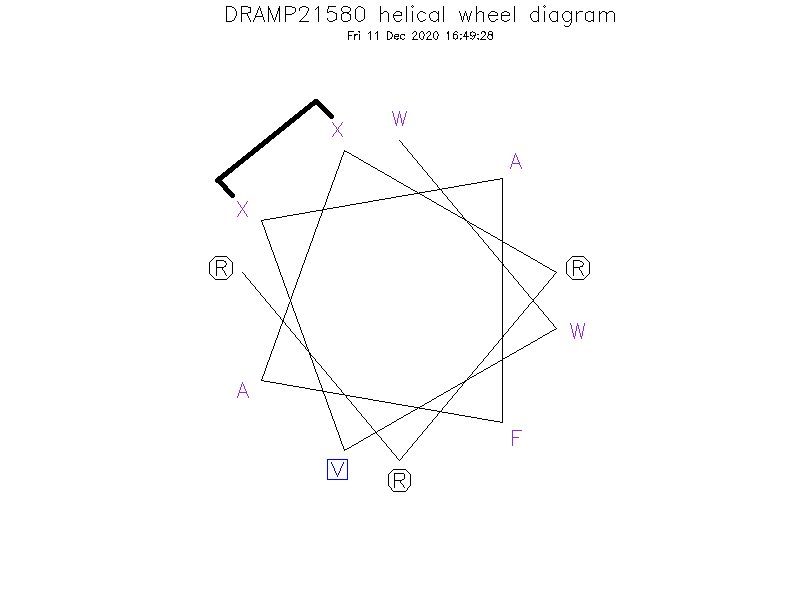
- WWVXAFAXRRR
-
Source
- Synthetic construct
-
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+
-
- Function: Antibacterial activity against Gram-positive bacteria. Antibacterial activity against Gram-negative bacteria and antifungal activity against Candida albicans are not noteable under 20 μM.
-
Target Organism
-
- [Ref.28073163] Gram-positive bacteria: Bacillus megaterium ATCC 14581 (IC50 = 1.63 μM, MIC = 5.00 μM), Staphylococcus aureus ATCC 6538 (IC50 = 0.64 μM, MIC = 5.00 μM), Enterococcus faecalis ATCC 29212 (IC50 = 0.63 μM, MIC = 1.25 μM);
- Gram-negative bacteria: Escherichia coli ATCC 700926 (IC50 = 169 μM, MIC > 20 μM);
- Fungi: Candida albicans 002 ATCC 64385 (IC50 > 20 μM, MIC > 20 μM), C. albicans 004 ATCC MYA-2876 (IC50 > 20 μM, MIC > 20 μM).
-
Hemolytic Activity
-
- [Ref.28073163] HC50 = 4.49 μM against human red blood cells. Note: HC50 is the half-maximal hemolytic concentration.
-
Cytotoxicity
-
No cytotoxicity information found in the reference(s) presented
-
Linear/Cyclic
- Cyclic (Stapled)
-
N-terminal Modification
- Acylation (Caproylamide)
-
C-terminal Modification
- Amidation
-
Special Amino Acid and Stapling Position
- ①The Ⓧ (position: 4 and 8) in sequence indicates (S)-2-(4'-pentenyl)-alanine. ②Ⓧ (4) and Ⓧ (8) are cross-linked by hydrocarbon stapling through an oct-4-enyl hydrocarbon staple.
-
-
Secondary Structure
- 0.3% α-helix and 31.9% β-strand in 20 mM potassium phosphate buffer; 0.7% α-helix and 40.0% β-strand in 20 mM potassium phosphate buffer made 30% in TFE.
-
Structure Description
- Only slghtly higher β-strand characteristics were observed for the hydrocarbon-stapled peptides in 30% TFE. Overall, the peptides showed some β-strand secondary structural characteristics and little α-helical content.
-
-
There is no predicted structure for DRAMP21580.
- Literature 1
-
Title
- Hydrocarbon-stapled lipopeptides exhibit selective antimicrobial activity
-
-
Reference
- Biopolymers. 2017 May;108(3). doi: 10.1002/bip.23006.
-
Author
- Zachary B Jenner, Christopher M Crittenden, Martín Gonzalez, Jennifer S Brodbelt, Kerry A Bruns