-
-
Peptide Name
- peptide 2 (derived from OH-CM6)
-
Sequence
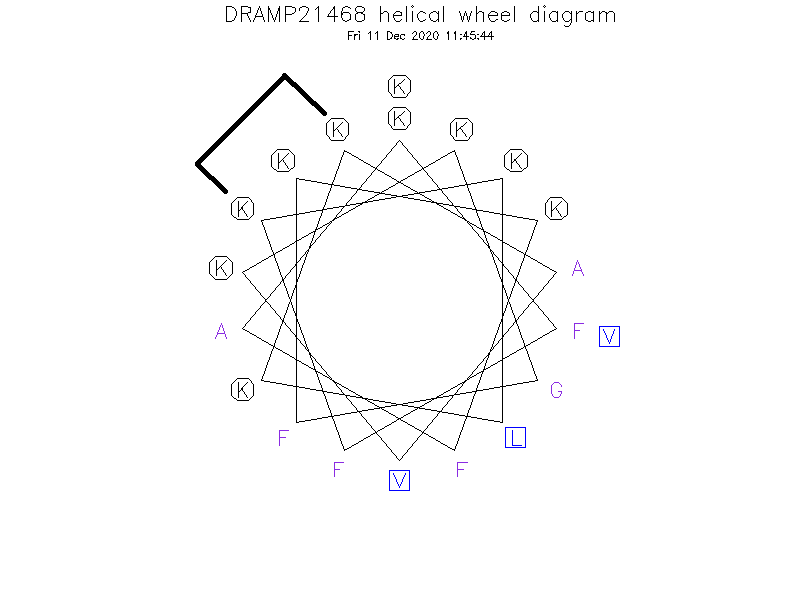
- KFFⓀKLKⓀAVKKGFKKFAKV
-
-
Original Sequence
- KFFKKLKKAVKKGFKKFAKV
-
Source
- Synthetic construct
-
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+, Anti-Gram-
-
- Function: Antibacterial activity against Gram-positive and Gram-negative bacteria.
-
Target Organism
-
- [Ref.32216308] Gram-positive bacteria: Staphylococcus aureus (MIC99.9= 8 μg/mL), methicillin-resistant Staphylococcus aureus (MIC99.9= 4 μg/mL), Listeria monocytogenes (MIC99.9= 2 μg/mL);
- Gram-negative bacteria: E.coli (MIC99.9= 16 μg/mL), Pseudomonas aeruginosa (MIC99.9= 8 μg/mL), clinically isolated drug-resistant E.coli (MIC99.9= 16 μg/mL)
-
Hemolytic Activity
-
- [Ref.32216308] It has 1.9% hemolysis against human red blood cells at peptide concentration of 320 μg/mL
-
Cytotoxicity
-
No cytotoxicity information found in the reference(s) presented
-
Linear/Cyclic
- Cyclic (Stapled)
-
N-terminal Modification
- Free
-
C-terminal Modification
- Amidation
-
Special Amino Acid and Stapling Position
- ①The Ⓚ (position: 4 and 8) in sequence indicates Nε-o-Ns-Nα-Fmoc-lysine before stapling. ②Ⓚ (4) and Ⓚ (8) are cross-linked by a (E)-but-2-enyl spacer employing the N-alkylation reaction.
-
-
Secondary Structure
- Random coils in PBS.
-
Structure Description
- ①All the peptides were random coils in PBS but displayed varied levels of α-helicity in the presence of 30 mM SDS. ②Other stapled peptides had an α-helix content ranging from 16 to 38%, but their antibacterial activity and proteolytic stability were quite similar.
-
-
There is no predicted structure for DRAMP21468.
- Literature 1
-
Title
- Novel Stapling by Lysine Tethering Provides Stable and Low Hemolytic Cationic Antimicrobial Peptides
-
-
Reference
- J Med Chem. 2020 Apr 23;63(8):4081-4089. doi: 10.1021/acs.jmedchem.9b02025. Epub 2020 Apr 8.
-
Author
- Hong Li, Yuchen Hu, Qi Pu, Tong He, Qianyu Zhang, Wen Wu, Xuefeng Xia and Jinqiang Zhang