General Information
-
DRAMP ID
- DRAMP21615
-
Peptide Name
- S6
-
Sequence
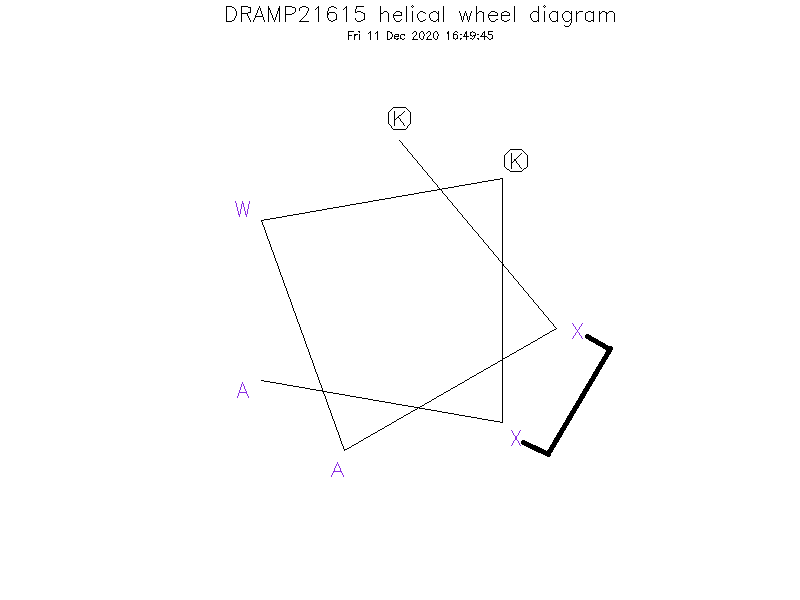
- KⓍAWKⓍA
-
Sequence Length
- 7
-
Original Sequence
- KXAWKXA
-
Source
- Synthetic construct
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram-
-
Comments
- Function: Antibacterial activity against Gram-negative bacteria. Antibacterial activity against Gram-positive bacteria is not noteable under 100 μM.
-
Target Organism
-
- Gram-positive bacteria: Bacillus subtilis ATCC 6633 (MIC > 100 μM), Staphylococcus aureus ATCC 6538p (MIC > 100 μM), Staphylococcus epidermis ATCC 12228 (MIC > 100 μM);
- Gram-negative bacteria: Escherichia coli ATCC 25922 (MIC = 100 μM), Shigella dysentariae ATCC 9752 (MIC > 100 μM), Salmonella typhimurium ATCC 14028 (MIC > 100 μM), Klebsiella pneumonia ATCC 10031 (MIC > 100 μM), Pseudomonas aeruginosa ATCC 27853 (MIC > 100 μM).
-
Hemolytic Activity
-
- It has 0.65%, 0.66%, 0.75%, 0.87%, 0.64%, 0.70%, 0.61% and 0.84% hemolysis against human red blood cells at 0.8, 1.6, 3.1, 6.3, 12.5, 25.0, 50.0 and 100.0 μM.
-
Cytotoxicity
- No cytotoxicity information found in the reference(s) presented
Structure Information
-
Linear/Cyclic
- Cyclic (Stapled)
-
N-terminal Modification
- Acetylation
-
C-terminal Modification
- Amidation
-
Special Amino Acid and Stapling Position
- ①The Ⓧ (position: 2 and 6) in sequence indicates (S)-α-methyl, α-pentenylglycine. Note: the Experimental section presenst that X is (S)-α-methyl, α-pentenylglycine, while the Results section presents that X is pentenylalanine. We incline to the former representation according to previous papers published by the research group which the author belonged. ②Ⓧ (2) and Ⓧ (6) are cross-linked by hydrocarbon stapling through an oct-4-enyl hydrocarbon staple.
-
Stereochemistry
- L
-
Secondary Structure
- α-helix in 25 mM potassium phosphate buffer solution (pH 6.5)
-
Structure Description
- As expected, in the far ultraviolet circular dichroism (CD) experiment, the stapled heptapeptides displayed enhanced helical contents compared to their corresponding unstapled counterparts.
-
Helical Wheel Diagram
-
Predicted Structure
- There is no predicted structure for DRAMP21615.
Physicochemical Information
-
Formula
- C₄₃H₆₈N₁₀O₈
Absent Amino Acids
- CDEFGHILMNPQRSTVY
Common Amino Acids
- AKX
Mass
- 853.09
PI
- /
-
Basic Residues
- 2
Acidic Residues
- 0
Hydrophobic Residues
- 3
Hydrophobicity
- /
Polar Residues
- 0
DRAMP21615
Literature Information
- Literature 1
-
Title
- De Novo Design and Their Antimicrobial Activity of Stapled Amphipathic Helices of Heptapeptides
-
Pubmed ID
- PubMed ID is not available
-
Reference
- B KOREAN CHEM SOC
-
Author
- Thuy T.T. Dinh, Do-Hee Kim, Song-Jin Lee, Young-Woo Kim