General Information
-
DRAMP ID
- DRAMP28998
-
Peptide Name
- Stapled heptapeptide 2
-
Sequence
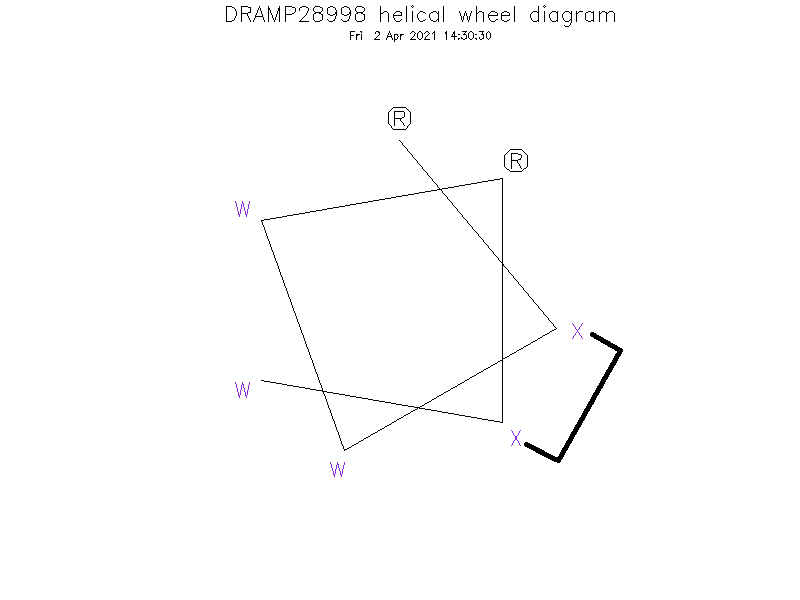
- RⓍWWRⓍW
-
Sequence Length
- 7
-
Original Sequence
- RWWWRWW
-
Source
- Synthetic construct
Activity Information
-
Biological Activity
- Antimicrobial, Antibacterial, Anti-Gram+
-
Comments
- Function: Antibacterial activity against Gram-positive bacteria. The stapled peptide was much more active against bacteria than the unstapled one.
-
Target Organism
-
- Gram-positive bacteria: Staphylococcus aureus ATCC25923 (MIC = 19 ± 4 μg/mL). Methicillin-resistant Staphylococcus aureus (MRSA) (MIC > 25 ± 6 μg/mL)
-
Hemolytic Activity
-
- No hemolytic activity information found.
-
Cytotoxicity
- No cytotoxicity information found in the reference(s) presented
Structure Information
-
Linear/Cyclic
- Cyclic (Stapled)
-
N-terminal Modification
- Free
-
C-terminal Modification
- Amidation
-
Special Amino Acid and Stapling Position
- ①The Ⓧ (position: 2 and 6) in sequence indicate (S)-4-pentenyl alanine. ②Ⓧ (2) and Ⓧ (6) are cross-linked by hydrocarbon stapling through an oct-4-enyl hydrocarbon staple.
-
Stereochemistry
- L
-
Secondary Structure
- Helicity = 61.3% in 10 mM sodium phosphate buffer (pH 7.4) at peptide concentrations of 100 μM
-
Structure Description
- CD spectroscopy was used to characterize the secondary structure of five unstapled heptapeptides and their stapled counterparts in phosphate buffer, indicating a significant increase in peptide helical content upon the stapling, with helicity change from h = 14.1% - 33.7% (for unstapled peptides) to h = 58.9%-75.1% (for stapled peptides).
-
Helical Wheel Diagram
-
Predicted Structure
- There is no predicted structure for DRAMP28998.
Physicochemical Information
-
Formula
- C₆₁H₈₂N₁₆O₈
Absent Amino Acids
- ACDEFGHIKLMNPQSTV
Common Amino Acids
- W
Mass
- 1167.4
PI
- /
-
Basic Residues
- 2
Acidic Residues
- 0
Hydrophobic Residues
- 3
Hydrophobicity
- /
Polar Residues
- 0
DRAMP28998
Literature Information
- Literature 1
-
Title
- De Novo Hydrocarbon-Stapling Design of Single-Turn α-Helical Antimicrobial Peptides
-
Pubmed ID
- PubMed ID is not available
-
Reference
- Int J Pept Res Ther. 2019 Nov 14; 26(4):1711–1719. doi: 10.1007/s10989-019-09964-7.
-
Author
- Zhixia Chen, Xiuli Yu, Aiying Zhang, Fangfang Wang, Yankun Xing